The Role of Intravitreal Corticosteroids in the Treatment of DME: Predictive OCT Biomarkers
- PMID: 35886930
- PMCID: PMC9319632
- DOI: 10.3390/ijms23147585
The Role of Intravitreal Corticosteroids in the Treatment of DME: Predictive OCT Biomarkers
Abstract
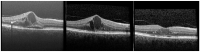
This work aims to summarize predictive biomarkers to guide treatment choice in DME. Intravitreal anti-VEGF is considered the gold standard treatment for centers involving DME, while intravitreal steroid treatment has been established as a second-line treatment in DME. However, more than 1/3 of the patients do not adequately respond to anti-VEGF treatment despite up to 4-weekly injections. Not surprisingly, insufficient response to anti-VEGF therapy has been linked to low-normal VEGF levels in the serum and aqueous humor. These patients may well benefit from an early switch to intravitreal steroid treatment. In these patients, morphological biomarkers visible in OCT may predict treatment response and guide treatment decisions. Namely, the presence of a large amount of retinal and choroidal hyperreflective foci, disruption of the outer retinal layers and other signs of chronicity such as intraretinal cysts extending into the outer retina and a lower choroidal vascular index are all signs suggestive of a favorable treatment response of steroids compared to anti-VEGF. This paper summarizes predictive biomarkers in DME in order to assist individual treatment decisions in DME. These markers will help to identify DME patients who may benefit from primary dexamethasone treatment or an early switch.
Keywords: DME; OCT; anti-VEGF; biomarker; corticosteroid; dexamethasone; diabetes mellitus; diabetic macular edema; diabetic maculopathy; response to treatment.
Conflict of interest statement
The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Marion R. Munk: Consultant: Novartis, Zeiss, Gensight Biologics, Lumithera, Bayer, Isarna Therapeutics, Roche, Allergan, RetinAI; Lecturer fees, Grant and travel support: Bayer, Allergan. Lala Ceklic: Consultant: Novartis, Bayer, Grant: Allergan. Justus G. Garweg: Consultant and Lecture fees: AbbVie, Alcon, Bayer, and Novartis, industry-sponsored clinical studies: Roche, Bayer, and Novartis. Gabor Mark Somfai: Consultant: Allergan, Bayer, Novartis, Carl Zeiss Meditec. Marcel N. Menke: Consultant and Lecture fees: Abbvie, Bayer, Novartis, Roche. Marc de Smet: no financial disclosures. Guy Donati: no financial disclosures.
Figures

References
-
- Holekamp N.M. Overview of diabetic macular edema. Am. J. Manag. Care. 2016;22:s284–s291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

