Light Triggers the Antiproliferative Activity of Naphthalimide-Conjugated (η6-arene)ruthenium(II) Complexes
- PMID: 35886972
- PMCID: PMC9322830
- DOI: 10.3390/ijms23147624
Light Triggers the Antiproliferative Activity of Naphthalimide-Conjugated (η6-arene)ruthenium(II) Complexes
Abstract
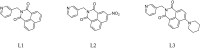
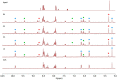
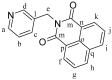
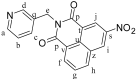
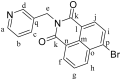
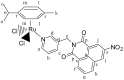
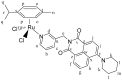
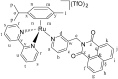
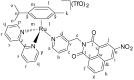
We report the synthesis and characterization of three half-sandwich Ru(II) arene complexes [(η6-arene)Ru(N,N')L][PF6]2 containing arene = p-cymene, N,N' = bipyridine, and L = pyridine meta- with methylenenaphthalimide (C1), methylene(nitro)naphthalimide (C2), or methylene(piperidinyl)naphthalimide (C3). The naphthalimide acts as an antenna for photoactivation. After 3 h of irradiation with blue light, the monodentate pyridyl ligand had almost completely dissociated from complex C3, which contains an electron donor on the naphthalimide ring, whereas only 50% dissociation was observed for C1 and C2. This correlates with the lower wavelength and strong absorption of C3 in this region of the spectrum (λmax = 418 nm) compared with C1 and C2 (λmax = 324 and 323 nm, respectively). All the complexes were relatively non-toxic towards A549 human lung cancer cells in the dark, but only complex C3 exhibited good photocytoxicity towards these cancer cells upon irradiation with blue light (IC50 = 10.55 ± 0.30 μM). Complex C3 has the potential for use in photoactivated chemotherapy (PACT).
Keywords: Ru(II) arene complexes; naphthalimide; photoactivated chemotherapy; photoactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
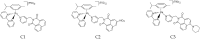



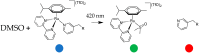



References
-
- Bacchi A., Capucci D., Gatti A., Loffi C., Pioli M., Rogolino D., Terenziani F., Pelagatti P. Synthesis, Structure and Spectroscopic Characterization of Halfsandwich Ru-II Complexes containing 1,8-Naphthalimide Ligands. ChemistrySelect. 2017;2:7000–7007. doi: 10.1002/slct.201701070. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

