Increased Acetylcholine Levels and Other Brain Effects in 5XFAD Mice after Treatment with 8,14-Dihydroxy Metabolite of Efavirenz
- PMID: 35887013
- PMCID: PMC9317559
- DOI: 10.3390/ijms23147669
Increased Acetylcholine Levels and Other Brain Effects in 5XFAD Mice after Treatment with 8,14-Dihydroxy Metabolite of Efavirenz
Abstract
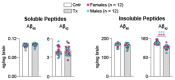
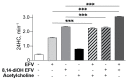
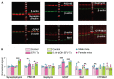
Efavirenz (EFV), an FDA-approved anti-HIV drug, has off-target binding to CYP46A1, the CNS enzyme which converts cholesterol to 24-hydroxycholesterol. At small doses, EFV allosterically activates CYP46A1 in mice and humans and mitigates some of the Alzheimer's disease manifestations in 5XFAD mice, an animal model. Notably, in vitro, all phase 1 EFV hydroxymetabolites activate CYP46A1 as well and bind either to the allosteric site for EFV, neurotransmitters or both. Herein, we treated 5XFAD mice with 8,14-dihydroxyEFV, the binder to the neurotransmitter allosteric site, which elicits the highest CYP46A1 activation in vitro. We found that treated animals of both sexes had activation of CYP46A1 and cholesterol turnover in the brain, decreased content of the amyloid beta 42 peptide, increased levels of acetyl-CoA and acetylcholine, and altered expression of the brain marker proteins. In addition, male mice had improved performance in the Barnes Maze test and increased expression of the acetylcholine-related genes. This work expands our knowledge of the beneficial CYP46A1 activation effects and demonstrates that 8,14-dihydroxyEFV crosses the blood-brain barrier and has therapeutic potential as a CYP46A1 activator.
Keywords: 8,14-dihydroxyefavirenz; Alzheimer’s disease; CYP46A1; acetyl-CoA; acetylcholine; cholesterol metabolism; efavirenz.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
7,8-Dihydroxy Efavirenz Is Not as Effective in CYP46A1 Activation In Vivo as Efavirenz or Its 8,14-Dihydroxy Metabolite.Int J Mol Sci. 2024 Feb 13;25(4):2242. doi: 10.3390/ijms25042242. Int J Mol Sci. 2024. PMID: 38396919 Free PMC article.
-
CYP46A1 Activation by Efavirenz Leads to Behavioral Improvement without Significant Changes in Amyloid Plaque Load in the Brain of 5XFAD Mice.Neurotherapeutics. 2019 Jul;16(3):710-724. doi: 10.1007/s13311-019-00737-0. Neurotherapeutics. 2019. PMID: 31062296 Free PMC article.
-
Cholesterol-metabolizing enzyme cytochrome P450 46A1 as a pharmacologic target for Alzheimer's disease.Neuropharmacology. 2017 Sep 1;123:465-476. doi: 10.1016/j.neuropharm.2017.06.026. Epub 2017 Jun 24. Neuropharmacology. 2017. PMID: 28655608 Free PMC article.
-
Cholesterol 24-Hydroxylation by CYP46A1: Benefits of Modulation for Brain Diseases.Neurotherapeutics. 2019 Jul;16(3):635-648. doi: 10.1007/s13311-019-00731-6. Neurotherapeutics. 2019. PMID: 31001737 Free PMC article. Review.
-
The potential of CYP46A1 as a novel therapeutic target for neurological disorders: An updated review of mechanisms.Eur J Pharmacol. 2023 Jun 15;949:175726. doi: 10.1016/j.ejphar.2023.175726. Epub 2023 Apr 14. Eur J Pharmacol. 2023. PMID: 37062503 Review.
Cited by
-
7,8-Dihydroxy Efavirenz Is Not as Effective in CYP46A1 Activation In Vivo as Efavirenz or Its 8,14-Dihydroxy Metabolite.Int J Mol Sci. 2024 Feb 13;25(4):2242. doi: 10.3390/ijms25042242. Int J Mol Sci. 2024. PMID: 38396919 Free PMC article.
-
CYP46A1 activation by low-dose efavirenz enhances brain cholesterol metabolism in subjects with early Alzheimer's disease.Alzheimers Res Ther. 2022 Dec 29;14(1):198. doi: 10.1186/s13195-022-01151-z. Alzheimers Res Ther. 2022. PMID: 36581878 Free PMC article. Clinical Trial.
-
Challenges and Opportunities for Consideration of Efavirenz Drug Repurposing for Alzheimer's Disease Therapeutics.ACS Pharmacol Transl Sci. 2024 Sep 6;7(10):2924-2935. doi: 10.1021/acsptsci.4c00229. eCollection 2024 Oct 11. ACS Pharmacol Transl Sci. 2024. PMID: 39421657 Free PMC article. Review.
References
-
- Hudry E., Van Dam D., Kulik W., De Deyn P.P., Stet F.S., Ahouansou O., Benraiss A., Delacourte A., Bougneres P., Aubourg P., et al. Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer’s disease. Mol. Ther. J. Am. Soc. Gene Ther. 2010;18:44–53. doi: 10.1038/mt.2009.175. - DOI - PMC - PubMed
-
- Burlot M.A., Braudeau J., Michaelsen-Preusse K., Potier B., Ayciriex S., Varin J., Gautier B., Djelti F., Audrain M., Dauphinot L., et al. Cholesterol 24-hydroxylase defect is implicated in memory impairments associated with Alzheimer-like Tau pathology. Hum. Mol. Genet. 2015;24:5965–5976. doi: 10.1093/hmg/ddv268. - DOI - PubMed
-
- Boussicault L., Alves S., Lamaziere A., Planques A., Heck N., Moumne L., Despres G., Bolte S., Hu A., Pages C., et al. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Pt 3Brain. 2016;139:953–970. doi: 10.1093/brain/awv384. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

