PAX Genes in Cardiovascular Development
- PMID: 35887061
- PMCID: PMC9324344
- DOI: 10.3390/ijms23147713
PAX Genes in Cardiovascular Development
Abstract
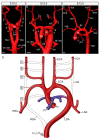
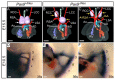
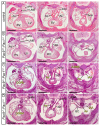
The mammalian heart is a four-chambered organ with systemic and pulmonary circulations to deliver oxygenated blood to the body, and a tightly regulated genetic network exists to shape normal development of the heart and its associated major arteries. A key process during cardiovascular morphogenesis is the septation of the outflow tract which initially forms as a single vessel before separating into the aorta and pulmonary trunk. The outflow tract connects to the aortic arch arteries which are derived from the pharyngeal arch arteries. Congenital heart defects are a major cause of death and morbidity and are frequently associated with a failure to deliver oxygenated blood to the body. The Pax transcription factor family is characterised through their highly conserved paired box and DNA binding domains and are crucial in organogenesis, regulating the development of a wide range of cells, organs and tissues including the cardiovascular system. Studies altering the expression of these genes in murine models, notably Pax3 and Pax9, have found a range of cardiovascular patterning abnormalities such as interruption of the aortic arch and common arterial trunk. This suggests that these Pax genes play a crucial role in the regulatory networks governing cardiovascular development.
Keywords: Pax3; Pax9; cardiovascular development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kelly R.G. Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension. Springer; Singapore: 2020. Advances in the Second Heart Field; pp. 301–307.
-
- Anderson R.H., Chaudhry B., Mohun T.J., Bamforth S.D., Hoyland D., Phillips H.M., Webb S., Moorman A.F., Brown N.A., Henderson D.J. Normal and abnormal development of the intrapericardial arterial trunks in humans and mice. Cardiovasc. Res. 2012;95:108–115. doi: 10.1093/cvr/cvs147. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

