Modelling the Human Blood-Brain Barrier in Huntington Disease
- PMID: 35887162
- PMCID: PMC9321930
- DOI: 10.3390/ijms23147813
Modelling the Human Blood-Brain Barrier in Huntington Disease
Abstract
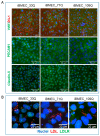
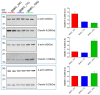
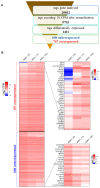
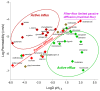
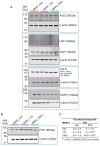
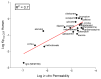
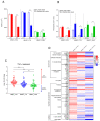
While blood-brain barrier (BBB) dysfunction has been described in neurological disorders, including Huntington's disease (HD), it is not known if endothelial cells themselves are functionally compromised when promoting BBB dysfunction. Furthermore, the underlying mechanisms of BBB dysfunction remain elusive given the limitations with mouse models and post mortem tissue to identify primary deficits. We established models of BBB and undertook a transcriptome and functional analysis of human induced pluripotent stem cell (iPSC)-derived brain-like microvascular endothelial cells (iBMEC) from HD patients or unaffected controls. We demonstrated that HD-iBMECs have abnormalities in barrier properties, as well as in specific BBB functions such as receptor-mediated transcytosis.
Keywords: Huntington’s disease; blood–brain barrier; brain endothelial cells; in vitro models; induced pluripotent stem cells; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Drouin-Ouellet J., Sawiak S., Cisbani G., Lagacé M., Kuan W.-L., Saint-Pierre M., Dury R.J., Alata W., St-Amour I., Mason S.L., et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann. Neurol. 2015;78:160–177. doi: 10.1002/ana.24406. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

