Differences in the Autophagy Response to Hypoxia in the Hippocampus and Neocortex of Rats
- PMID: 35887346
- PMCID: PMC9320385
- DOI: 10.3390/ijms23148002
Differences in the Autophagy Response to Hypoxia in the Hippocampus and Neocortex of Rats
Abstract
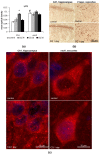
Autophagy is a regulated mechanism of degradation of misfolded proteins and organelles in the cell. Neurons are highly differentiated cells with extended projections, and therefore, their functioning largely depends on the mechanisms of autophagy. For the first time in an animal model using immunohistochemistry, dot analysis, and qRT-PCR, the autophagy (macroautophagy) activity in neurons of two brain regions (hippocampus and neocortex) under normoxia and after exposure to hypoxia was studied. It was found that under normoxia, the autophagic activity was higher in the hippocampal neurons than in the neocortex of rats. In the hippocampus, the exposure of rats to hypoxia resulted in a decrease in the content of autophagy markers LC3 and p62, which was followed by activation of the autophagy-related gene expression. In the neocortex, no changes in these marker proteins were observed after the exposure to hypoxia. These data indicate that the neurons in the hippocampus and neocortex differ in the autophagy response to hypoxia, which may reflect the physiological and functional differences of the pyramidal cells of these brain regions and may to some extent account for the extreme vulnerability of the CA1 hippocampal neurons and relatively high resistance of the neocortical neurons to hypoxia.
Keywords: autophagy; hippocampus; hypoxia; neocortex; neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

