Perivascular Mesenchymal Stem/Stromal Cells, an Immune Privileged Niche for Viruses?
- PMID: 35887383
- PMCID: PMC9317325
- DOI: 10.3390/ijms23148038
Perivascular Mesenchymal Stem/Stromal Cells, an Immune Privileged Niche for Viruses?
Abstract
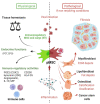
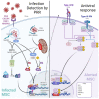
Mesenchymal stem cells (MSCs) play a critical role in response to stress such as infection. They initiate the removal of cell debris, exert major immunoregulatory activities, control pathogens, and lead to a remodeling/scarring phase. Thus, host-derived 'danger' factors released from damaged/infected cells (called alarmins, e.g., HMGB1, ATP, DNA) as well as pathogen-associated molecular patterns (LPS, single strand RNA) can activate MSCs located in the parenchyma and around vessels to upregulate the expression of growth factors and chemoattractant molecules that influence immune cell recruitment and stem cell mobilization. MSC, in an ultimate contribution to tissue repair, may also directly trans- or de-differentiate into specific cellular phenotypes such as osteoblasts, chondrocytes, lipofibroblasts, myofibroblasts, Schwann cells, and they may somehow recapitulate their neural crest embryonic origin. Failure to terminate such repair processes induces pathological scarring, termed fibrosis, or vascular calcification. Interestingly, many viruses and particularly those associated to chronic infection and inflammation may hijack and polarize MSC's immune regulatory activities. Several reports argue that MSC may constitute immune privileged sanctuaries for viruses and contributing to long-lasting effects posing infectious challenges, such as viruses rebounding in immunocompromised patients or following regenerative medicine therapies using MSC. We will herein review the capacity of several viruses not only to infect but also to polarize directly or indirectly the functions of MSC (immunoregulation, differentiation potential, and tissue repair) in clinical settings.
Keywords: COVID-19; chikungunya; chronic inflammation; fibroblast; immune-regulation; immunity; innate immunity; mesenchymal stem cells; neural crest; pericytes; persistence; stromal cells; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

