Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans
- PMID: 35887511
- PMCID: PMC9325280
- DOI: 10.3390/jof8070756
Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans
Abstract
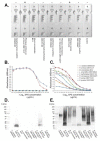
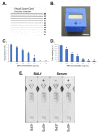
Mucormycosis is a highly aggressive angio-invasive disease of humans caused by fungi in the zygomycete order, Mucorales. Though a number of different species can cause mucormycosis, the principal agent of the disease worldwide is Rhizopus arrhizus, which accounts for the majority of rhino-orbital-cerebral, pulmonary, and disseminated infections in immunocompromised individuals. It is also the main cause of life-threatening infections in patients with poorly controlled diabetes mellitus, and in corticosteroid-treated patients with SARS-CoV-2 infection, where it causes the newly described disease, COVID-19-associated mucormycosis (CAM). Diagnosis currently relies on non-specific CT, a lengthy and insensitive culture from invasive biopsy, and a time-consuming histopathology of tissue samples. At present, there are no rapid antigen tests for the disease that detect biomarkers of infection, and which allow point-of-care diagnosis. Here, we report the development of an IgG1 monoclonal antibody (mAb), KC9, which is specific to Rhizopus arrhizus var. arrhizus (syn. Rhizopus oryzae) and Rhizopus arrhizus var. delemar (Rhizopus delemar), and which binds to a 15 kDa extracellular polysaccharide (EPS) antigen secreted during hyphal growth of the pathogen. Using the mAb, we have developed a competitive lateral-flow device (LFD) that allows rapid (30 min) and sensitive (~50 ng/mL running buffer) detection of the EPS biomarker, and which is compatible with human serum (limit of detection of ~500 ng/mL) and bronchoalveolar lavage fluid (limit of detection of ~100 ng/mL). The LFD, therefore, provides a potential novel opportunity for the non-invasive detection of mucormycosis caused by Rhizopus arrhizus.
Keywords: Rhizopus; biomarker; lateral-flow device; monoclonal antibody; mucormycosis.
Conflict of interest statement
CRT is a Director of ISCA Diagnostics Ltd. This manuscript does not have any potential conflict of interest with ISCA Diagnostics Ltd. The other authors declare no conflicts of interest.
Figures

Similar articles
-
Development of a monoclonal antibody and a lateral-flow device for the rapid detection of a Mucorales-specific biomarker.Front Cell Infect Microbiol. 2023 Dec 8;13:1305662. doi: 10.3389/fcimb.2023.1305662. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38145040 Free PMC article.
-
A Lateral-Flow Device for the Rapid Detection of Scedosporium Species.Diagnostics (Basel). 2024 Apr 19;14(8):847. doi: 10.3390/diagnostics14080847. Diagnostics (Basel). 2024. PMID: 38667492 Free PMC article.
-
Development and evaluation of a Pan-Mucorales Real-time PCR and a multiplex Real-time PCR for detection and identification of Rhizopus arrhizus, Rhizopus microsporus, and Mucor spp. in clinical specimens.J Clin Microbiol. 2025 Jun 11;63(6):e0193724. doi: 10.1128/jcm.01937-24. Epub 2025 Apr 30. J Clin Microbiol. 2025. PMID: 40304523 Free PMC article.
-
Mucormycosis in Africa: Epidemiology, diagnosis and treatment outcomes.Mycoses. 2023 Jul;66(7):555-562. doi: 10.1111/myc.13581. Epub 2023 Mar 7. Mycoses. 2023. PMID: 36856432 Review.
-
Multidisciplinary approach in diagnosis and treatment of COVID-19-associated mucormycosis: a description of current reports.Egypt J Intern Med. 2022;34(1):58. doi: 10.1186/s43162-022-00143-7. Epub 2022 Jul 23. Egypt J Intern Med. 2022. PMID: 35911783 Free PMC article. Review.
Cited by
-
Current Issues in Fungal Infections and COVID-19.J Fungi (Basel). 2022 Oct 23;8(11):1115. doi: 10.3390/jof8111115. J Fungi (Basel). 2022. PMID: 36354882 Free PMC article.
-
Fungal Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management.Clin Microbiol Rev. 2023 Sep 21;36(3):e0001923. doi: 10.1128/cmr.00019-23. Epub 2023 Jul 13. Clin Microbiol Rev. 2023. PMID: 37439685 Free PMC article. Review.
-
ESCMID-EFISG Survey on Diagnostic and Therapeutic Capacity for Invasive Fungal Infections in Belgium, the Netherlands, and Luxembourg: A Focus on High Azole Resistance.Mycoses. 2025 Jul;68(7):e70092. doi: 10.1111/myc.70092. Mycoses. 2025. PMID: 40650433 Free PMC article.
-
A Multiplex PCR and DNA-Sequencing Workflow on Serum for the Diagnosis and Species Identification for Invasive Aspergillosis and Mucormycosis.J Clin Microbiol. 2023 Jan 26;61(1):e0140922. doi: 10.1128/jcm.01409-22. Epub 2022 Dec 19. J Clin Microbiol. 2023. PMID: 36533925 Free PMC article.
-
Bacterial co-infections in mucormycosis in severely ill populations: an overlooked and complex challenge.Access Microbiol. 2024 Nov 12;6(11):000850.v4. doi: 10.1099/acmi.0.000850.v4. eCollection 2024. Access Microbiol. 2024. PMID: 39534303 Free PMC article.
References
-
- Thornton C.R. Detection of the ‘big five’ mold killers of humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes. Adv. Appl. Microbiol. 2020;110:1–61. - PubMed
-
- Diwakar J., Sammadar A., Konar S.K., Bhat M.D., Manuel E., Veenakumari H.B., Nandeesh B.N., Parveen A., Hajira S.N., Srinivas D., et al. First report of COVID-19-assciated rhino-orbital-cerebral mucormycosis in paediatric patients with type 1 diabetes mellitus. J. Med. Mycol. 2021;31:101203. doi: 10.1016/j.mycmed.2021.101203. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

