Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma
- PMID: 35887583
- PMCID: PMC9324584
- DOI: 10.3390/jpm12071086
Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma
Abstract
The prognosis of cholangiocarcinoma remains poor in spite of the advances in immunotherapy and molecular profiling, which has led to the identification of several targetable genetic alterations. Surgical procedures, including both liver resection and liver transplantation, still represent the treatment with the best curative potential, though the outcomes are significantly compromised by the early development of lymph node metastases. Progression of lymphatic metastasis from the primary tumor to tumor-draining lymph nodes is mediated by tumor-associated lymphangiogenesis, a topic largely overlooked until recently. Recent findings highlight tumor-associated lymphangiogenesis as paradigmatic of the role played by the tumor microenvironment in sustaining cholangiocarcinoma invasiveness and progression. This study reviews the current knowledge about the intercellular signaling and molecular mechanism of tumor-associated lymphangiogenesis in cholangiocarcinoma in the hope of identifying novel therapeutic targets to halt a process that often limits the success of the few available treatments.
Keywords: VEGF-C; VEGFR-3; biliary neoplasia; cancer-associated fibroblasts; lymphatic vessel; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
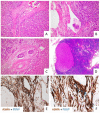
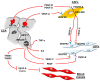
References
-
- Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. - DOI - PMC - PubMed
-
- Rizzo A., Ricci D.A., Brandi G. Detecting and targeting NTRK gene fusions in cholangiocarcinoma: News and perspectives. Expert Rev. Precis. Med. Drug Dev. 2021;6:225–227. doi: 10.1080/23808993.2021.1910023. - DOI
-
- Tawarungruang C., Khuntikeo N., Chamadol N., Laopaiboon V., Thuanman J., Thinkhamrop K., Kelly M., Thinkhamrop B. Survival after surgery among patients with cholangiocarcinoma in Northeast Thailand according to anatomical and morphological classification. BMC Cancer. 2021;21:497. doi: 10.1186/s12885-021-08247-z. - DOI - PMC - PubMed
-
- Izquierdo-Sanchez L., Lamarca A., La Casta A., Buettner S., Utpatel K., Klümpen H.J., Adeva J., Vogel A., Lleo A., Fabris L., et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022;76:1109–1121. doi: 10.1016/j.jhep.2021.12.010. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

