Increased Risk of NAFLD in Adults with Glomerular Hyperfiltration: An 8-Year Cohort Study Based on 147,162 Koreans
- PMID: 35887639
- PMCID: PMC9320347
- DOI: 10.3390/jpm12071142
Increased Risk of NAFLD in Adults with Glomerular Hyperfiltration: An 8-Year Cohort Study Based on 147,162 Koreans
Abstract
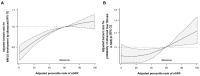
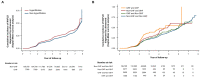
This study evaluated whether glomerular hyperfiltration (GHF) could predict nonalcoholic fatty liver disease (NAFLD) and fibrosis. A longitudinal cohort study including 147,479 participants aged 20-65 years without NAFLD and kidney disease at baseline was performed. GHF cutoff values were defined as age- and sex-specific estimated glomerular filtration rate (eGFRs) above the 95th percentile, and eGFR values between the 50th and 65th percentiles were used as reference groups. NAFLD was diagnosed via abdominal ultrasonography, and the fibrosis status was evaluated using the NAFLD fibrosis score and Fibrosis-4. During 598,745 person years of follow-up (median, 4.6 years), subjects with GHF at baseline had the highest hazard ratio (HR) for the development of NAFLD (HR 1.21; 95% CI 1.14-1.29) and fibrosis progression (HR 1.42; 95% CI 1.11-1.82) after adjusting for confounding factors. A higher baseline eGFR percentile maintained a higher risk of NAFLD and fibrosis probability. The persistent GHF group during follow-up had the highest HR for NAFLD compared to the persistent non-GHF group (HR 1.31; 95% CI 1.14-1.51). These results were consistent in all subgroups and statistically more prominent in participants without diabetes. GHF was positively associated with increased risk of NAFLD and probability of liver fibrosis in healthy adults.
Keywords: cohort study; glomerular filtration rate; insulin resistance; liver fibrosis; nonalcoholic fatty liver disease; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Baseline homeostasis model assessment of insulin resistance associated with fibrosis progression in patients with nonalcoholic fatty liver disease without diabetes: A cohort study.PLoS One. 2021 Aug 25;16(8):e0255535. doi: 10.1371/journal.pone.0255535. eCollection 2021. PLoS One. 2021. PMID: 34432804 Free PMC article.
-
Role of Heart Rate Variability in Association Between Glomerular Hyperfiltration and All-Cause Mortality.J Am Heart Assoc. 2021 Dec 21;10(24):e021585. doi: 10.1161/JAHA.121.021585. Epub 2021 Dec 10. J Am Heart Assoc. 2021. PMID: 34889105 Free PMC article.
-
New Nonalcoholic Fatty Liver Disease and Fibrosis Progression Associate With the Risk of Incident Chronic Kidney Disease.J Clin Endocrinol Metab. 2021 Sep 27;106(10):e3957-e3968. doi: 10.1210/clinem/dgab425. J Clin Endocrinol Metab. 2021. PMID: 34125886
-
Association of metabolic traits with occurrence of nonalcoholic fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis of longitudinal cohort studies.Saudi J Gastroenterol. 2022 Mar-Apr;28(2):92-100. doi: 10.4103/sjg.sjg_260_21. Saudi J Gastroenterol. 2022. PMID: 34810377 Free PMC article.
-
Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis.Gastroenterology. 2020 May;158(6):1611-1625.e12. doi: 10.1053/j.gastro.2020.01.043. Epub 2020 Feb 4. Gastroenterology. 2020. PMID: 32027911
Cited by
-
Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective.Diabetes Metab J. 2024 May;48(3):327-339. doi: 10.4093/dmj.2023.0350. Epub 2024 Feb 2. Diabetes Metab J. 2024. PMID: 38310873 Free PMC article. Review.
-
Glomerular Hyperfiltration: A Marker of Fibrosis Severity in Metabolic Associated Steatotic Liver Disease in an Adult Population.Int J Mol Sci. 2023 Oct 31;24(21):15837. doi: 10.3390/ijms242115837. Int J Mol Sci. 2023. PMID: 37958820 Free PMC article.
References
-
- Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

