Doripenem in the Treatment of Patients with Nosocomial Pneumonia: A Meta-Analysis
- PMID: 35887777
- PMCID: PMC9319354
- DOI: 10.3390/jcm11144014
Doripenem in the Treatment of Patients with Nosocomial Pneumonia: A Meta-Analysis
Abstract
Introduction: Clinically, doripenem therapy for nosocomial pneumonia remains a serious concern. The purpose of this meta-analysis was to explore the efficacy and the safety of doripenem therapy for nosocomial pneumonia in comparison with other antimicrobial agents.
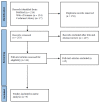
Methods: Studies were eligible for inclusion only if they directly compared the clinical effectiveness of doripenem and other antimicrobial agent therapies for nosocomial pneumonia in adult patients between 1 January 2000 and 30 April 2022. All studies were included if they reported one or more of the following outcomes: clinical cure rate, microbiological cure rate, all-cause mortality, and adverse events.
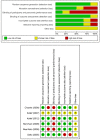
Results: Six randomized controlled trials and three retrospective studies were included in the meta-analysis. There were 952 patients in the doripenem group and 1183 patients in the comparator group. The comparator antimicrobial agents included imipenem/cilastatin, meropenem, and piperacillin/tazobactam. Seven studies had a high risk of bias. Doripenem therapy for nosocomial pneumonia had a microbiological cure rate, a clinical cure rate, an all-cause mortality, and adverse events similar to those of comparators.
Conclusions: The efficacy and the safety of doripenem therapy for nosocomial pneumonia were comparable with those of comparators. Randomized controlled trials are needed to confirm the role of doripenem in nosocomial pneumonia therapy.
Keywords: Pseudomonas aeruginosa; doripenem; hospital-acquired pneumonia; ventilator-associated pneumonia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




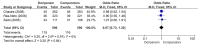
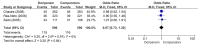


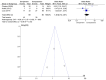
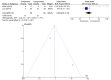
Similar articles
-
Medical resource utilization among patients with ventilator-associated pneumonia: pooled analysis of randomized studies of doripenem versus comparators.Crit Care. 2010;14(3):R84. doi: 10.1186/cc9012. Epub 2010 May 10. Crit Care. 2010. PMID: 20459721 Free PMC article.
-
Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter study.Curr Med Res Opin. 2008 Jul;24(7):2113-26. doi: 10.1185/03007990802179255. Epub 2008 Jun 11. Curr Med Res Opin. 2008. PMID: 18549664 Clinical Trial.
-
Meta-analysis of doripenem vs comparators in patients with pseudomonas infections enrolled in four phase III efficacy and safety clinical trials.Curr Med Res Opin. 2009 Dec;25(12):3029-36. doi: 10.1185/03007990903396790. Curr Med Res Opin. 2009. PMID: 19849650
-
Comparative review of the carbapenems.Drugs. 2007;67(7):1027-52. doi: 10.2165/00003495-200767070-00006. Drugs. 2007. PMID: 17488146 Review.
-
Doripenem monohydrate, a broad-spectrum carbapenem antibiotic.Clin Ther. 2009 Jan;31(1):42-63. doi: 10.1016/j.clinthera.2009.01.013. Clin Ther. 2009. PMID: 19243706 Review.
Cited by
-
Comparing the Outcomes of Ceftaroline plus Vancomycin or Daptomycin Combination Therapy versus Vancomycin or Daptomycin Monotherapy in Adults with Methicillin-Resistant Staphylococcus aureus Bacteremia-A Meta-Analysis.Antibiotics (Basel). 2022 Aug 15;11(8):1104. doi: 10.3390/antibiotics11081104. Antibiotics (Basel). 2022. PMID: 36009973 Free PMC article. Review.
-
The efficacy of N-acetylcysteine in chronic obstructive pulmonary disease patients: a meta-analysis.Ther Adv Respir Dis. 2023 Jan-Dec;17:17534666231158563. doi: 10.1177/17534666231158563. Ther Adv Respir Dis. 2023. PMID: 36927162 Free PMC article.
References
-
- Jones R.N., Huynh H.K., Biedenbach D.J., Fritsche T.R., Sader H.S. Doripenem (s-4661), a novel carbapenem: Comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. J. Antimicrob. Chemother. 2004;54:144–154. doi: 10.1093/jac/dkh298. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

