Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases
- PMID: 35887883
- PMCID: PMC9320118
- DOI: 10.3390/jcm11144119
Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases
Abstract
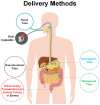
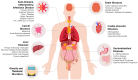
The human body is home to a variety of micro-organisms. Most of these microbial communities reside in the gut and are referred to as gut microbiota. Over the last decades, compelling evidence showed that a number of human pathologies are associated with microbiota dysbiosis, thereby suggesting that the reinstatement of physiological microflora balance and composition might ameliorate the clinical symptoms. Among possible microbiota-targeted interventions, pre/pro-biotics supplementations were shown to provide effective results, but the main limitation remains in the limited microbial species available as probiotics. Differently, fecal microbiota transplantation involves the transplantation of a solution of fecal matter from a donor into the intestinal tract of a recipient in order to directly change the recipient's gut microbial composition aiming to confer a health benefit. Firstly used in the 4th century in traditional Chinese medicine, nowadays, it has been exploited so far to treat recurrent Clostridioides difficile infections, but accumulating data coming from a number of clinical trials clearly indicate that fecal microbiota transplantation may also carry the therapeutic potential for a number of other conditions ranging from gastrointestinal to liver diseases, from cancer to inflammatory, infectious, autoimmune diseases and brain disorders, obesity, and metabolic syndrome. In this review, we will summarize the commonly used preparation and delivery methods, comprehensively review the evidence obtained in clinical trials in different human conditions and discuss the variability in the results and the pivotal importance of donor selection. The final aim is to stimulate discussion and open new therapeutic perspectives among experts in the use of fecal microbiota transplantation not only in Clostridioides difficile infection but as one of the first strategies to be used to ameliorate a number of human conditions.
Keywords: fecal microbiota transplantation; gut microbiome; neurological disorders.
Conflict of interest statement
M.B. is CEO of The Bioarte Ltd. G.D. declares no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

