Mucosal Eosinophil Abundance in Non-Inflamed Colonic Tissue Is Associated with Response to Vedolizumab Induction Therapy in Inflammatory Bowel Disease
- PMID: 35887905
- PMCID: PMC9318498
- DOI: 10.3390/jcm11144141
Mucosal Eosinophil Abundance in Non-Inflamed Colonic Tissue Is Associated with Response to Vedolizumab Induction Therapy in Inflammatory Bowel Disease
Abstract
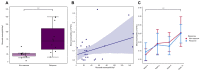
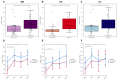
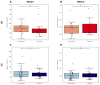
Vedolizumab is used as a treatment for patients with inflammatory bowel disease (IBD), but induction therapy leads to clinical response and remission in approximately 55% and 30% of patients with IBD, respectively. In this study, we aimed to explore the predictive value of mucosal eosinophils and serum eotaxin-1 regarding response to vedolizumab induction therapy. Eighty-four (84) patients with IBD (37 Crohn’s disease [CD], 47 ulcerative colitis [UC]) were included. For 24 patients with IBD, histopathology was assessed for eosinophil counts in non-inflamed colonic tissue prior to vedolizumab treatment. For 64 patients with IBD, serum eotaxin-1 levels were quantified prior to (baseline) and during vedolizumab treatment. Serum samples of 100 patients with IBD (34 CD, 66 UC) from the GEMINI 1 and 2 trials were used for external validation. Baseline mucosal eosinophil numbers in non-inflamed colonic tissue were significantly higher in responders to vedolizumab induction therapy when compared to primary non-responders (69 [34−138] vs. 24 [18−28] eosinophils/high-power field, respectively, p < 0.01). Baseline serum eotaxin-1 levels in the discovery cohort were significantly elevated in responders, compared to primary non-responders (0.33 [0.23−0.44] vs. 0.20 [0.16−0.29] ng/mL, p < 0.01). Prediction models based on mucosal eosinophil counts and serum eotaxin-1 showed an area under the curve (AUC) of 0.90 and 0.79, respectively. However, the predictive capacity of baseline serum eotaxin-1 levels could not be validated in the GEMINI cohort. Mucosal eosinophil abundance in non-inflamed colonic tissue was associated with response to vedolizumab induction therapy in patients with IBD. Future studies are warranted to further validate the potential value of mucosal eosinophils and serum eotaxin-1 as biomarkers for response to vedolizumab therapy.
Keywords: eosinophil; eotaxin-1; inflammatory bowel disease; vedolizumab.
Conflict of interest statement
G.D. received research grants from Royal DSM and speaker’s fees from Janssen Pharmaceuticals, Takeda, Pfizer and Abbvie. R.K.W. acted as consultant for Takeda, received unrestricted research grants from Takeda, Johnson & Johnson, Tramedico and Ferring, and received speaker’s fees from MSD, Abbvie and Janssen Pharmaceuticals. All other authors have no conflict of interest to declare.
Figures

References
-
- Kornbluth A., Sachar D.B., Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. - DOI - PubMed
-
- Feagan B.G., Greenberg G.R., Wild G., Fedorak R.N., Pare P., McDonald J.W., Cohen A., Bitton A., Baker A., Dubé R., et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin. Gastroenterol. Hepatol. 2008;6:1370–1377. doi: 10.1016/j.cgh.2008.06.007. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

