Dabigatran in Cerebral Sinus Vein Thrombosis and Thrombophilia
- PMID: 35888060
- PMCID: PMC9316430
- DOI: 10.3390/life12070970
Dabigatran in Cerebral Sinus Vein Thrombosis and Thrombophilia
Abstract
Background and purpose: Thrombophilic gene alterations are a major risk factor for cerebral sinus vein thrombosis (CSVT). Up to 30% of all patients with cerebral sinus vein thrombosis (CSVT) are found to have thrombophilic defects such as prothrombin mutation (PTM) or factor V Leiden (FVL). Their repercussions on the plasma levels of dabigatran etexilate are unclear. In this prospective case-control study, we aimed to investigate whether thrombophilia in CSVT has an influence on dabigatran peak-plasma levels.
Methods: We monitored 10 patients over 12 months with acute CSVT, genetic thrombophilia with off-label use of dabigatran etexilate 150 mg twice a day and measured dabigatran peak-plasma levels and radiological outcome. We also monitored patients without genetic thrombophilia with dabigatran etexilate 150 mg twice a day and compared the efficiency and dabigatran peak-plasma levels.
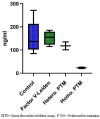
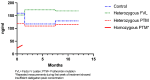
Results: Patients with homozygote PTM had significantly lower dabigatran peak concentration compared to patients with FVL or the control group (23 ± 4.2 vs. 152.3 ± 27.5 and 159.6 ± 63.08; p-value ≤ 0.05) There was no significant difference in dabigatran etexilate plasma levels between the heterozygote PTM group compared to patients with FVL or the control group (p = 0.29). There was no correlation between dabigatran peak concentration and delayed thrombus dissolution.
Conclusions: Dabigatran peak concentration was stable in patients with heterozygote FVL and heterozygote PTM, but not in homozygote PTM, compared to controls. Genetic screening for thrombophilia in patients after CSVT may be useful to make patient tailored therapeutic decisions regarding oral anticoagulation and may decrease thrombotic events.
Keywords: cerebral sinus vein thrombosis; dabigatran peak concentration; factor II mutation; factor V Leiden; thrombus dissolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ferro J.M., Canhao P., Stam J., Bousser M.G., Barinagarrementeria F., Investigators I. Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. - DOI - PubMed
LinkOut - more resources
Full Text Sources