Homozygous Pro1066Arg MYBPC3 Pathogenic Variant in a 26Mb Region of Homozygosity Associated with Severe Hypertrophic Cardiomyopathy in a Patient of an Apparent Non-Consanguineous Family
- PMID: 35888124
- PMCID: PMC9316903
- DOI: 10.3390/life12071035
Homozygous Pro1066Arg MYBPC3 Pathogenic Variant in a 26Mb Region of Homozygosity Associated with Severe Hypertrophic Cardiomyopathy in a Patient of an Apparent Non-Consanguineous Family
Abstract
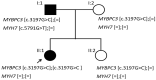
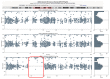
MYPBC3 and MYH7 are the most frequently mutated genes in patients with hereditary HCM. Homozygous and compound heterozygous genotypes generate the most severe phenotypes. A 35-year-old woman who was a homozygous carrier of the p.(Pro1066Arg) variant in the MYBPC3 gene, developed HCM phenocopy associated with left ventricular noncompaction and various degrees of conduction disease. Her father, a double heterozygote for this variant in MYBPC3 combined with the variant p.(Gly1931Cys) in the MYH7 gene, was affected by HCM. The variant in MYBPC3 in the heterozygosis-produced phenotype was neither in the mother nor in her only sister. Familial segregation analysis showed that the homozygous genotype p.(Pro1066Arg) was located in a region of 26 Mb loss of heterozygosity due to some consanguinity in the parents. These findings describe the pathogenicity of this variant, supporting the hypothesis of cumulative variants in cardiomyopathies, as well as the modulatory effect of the phenotype by other genes such as MYH7. Advancing HPO phenotyping promoted by the Human Phenotype Ontology, the gene-disease correlation, and vice versa, is evidence for the phenotypic heterogeneity of familial heart disease. The progressive establishment of phenotypic characteristics over time also complicates the clinical description.
Keywords: HPO terms; MYBPC3; MYH7; cardiac phenotype; region of homozygosity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Maron B.J., Gardin J.M., Flack J.M., Gidding S.S., Kurosaki T.T., Bild D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation. 1995;92:785–789. doi: 10.1161/01.CIR.92.4.785. - DOI - PubMed
-
- Richard P., Charron P., Carrier L., Ledeuil C., Cheav T., Pichereau C., Benaiche A., Isnard R., Dubourg O., Burban M., et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

