Biochemical Properties of Atranorin-Induced Behavioral and Systematic Changes of Laboratory Rats
- PMID: 35888178
- PMCID: PMC9316313
- DOI: 10.3390/life12071090
Biochemical Properties of Atranorin-Induced Behavioral and Systematic Changes of Laboratory Rats
Abstract
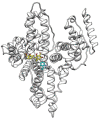
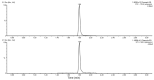
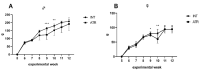
Atranorin (ATR) is a secondary metabolite of lichens. While previous studies investigated the effects of this substance predominantly in an in vitro environment, in our study we investigated the basic physicochemical properties, the binding affinity to human serum albumin (HSA), basic pharmacokinetics, and, mainly, on the systematic effects of ATR in vivo. Sporadic studies describe its effects during, predominantly, cancer. This project is original in terms of testing the efficacy of ATR on a healthy organism, where we can possibly attribute negative effects directly to ATR and not to the disease. For the experiment, 24 Sprague Dawley rats (Velaz, Únetice, Czech Republic) were used. The animals were divided into four groups. The first group (n = 6) included healthy males as control intact rats (♂INT) and the second group (n = 6) included healthy females as control intact rats (♀INT). Groups three and four (♂ATR/n = 6 and ♀ATR/n = 6) consisted of animals with daily administered ATR (10mg/kg body weight) in an ethanol-water solution per os for a one-month period. Our results demonstrate that ATR binds to HSA near the binding site TRP214 and acts on a systemic level. ATR caused mild anemia during the treatment. However, based on the levels of hepatic enzymes in the blood (ALT, ALP, or bilirubin levels), thiobarbituric acid reactive substances (TBARS), or liver histology, no impact on liver was recorded. Significantly increased creatinine and lactate dehydrogenase levels together with increased defecation activity during behavioral testing may indicate the anabolic effect of ATR in skeletal muscles. Interestingly, ATR changed some forms of behavior. ATR at a dose of 10 mg/kg body weight is non-toxic and, therefore, could be used in further research.
Keywords: atranorin; behavioral changes; human serum albumin; laboratory rats; metabolomics; microsomal stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Crawford S. Lichen Secondary Metabolites. Springer; Cham, Switzerland: 2015. Lichens Used in Traditional Medicine; pp. 27–80. - DOI
-
- Ranković B., Mišić M., Sukdolak S. The antimicrobial activity of substances derived from the lichens Physcia aipolia, Umbilicaria polyphylla, Parmelia caperata and Hypogymnia physodes. World J. Microbiol. Biotechnol. 2008;24:1239–1242. doi: 10.1007/s11274-007-9580-7. - DOI
-
- Verma N., Behera B., Parizadeh H., Sharma B. Bactericidal Activity of Some Lichen Secondary Compounds of Cladonia ochrochlora, Parmotrema nilgherrensis & Parmotrema sancti-angelii. Int. J. Drug Dev. Res. 2011;3:222–232.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

