Application of Genetically Encoded Photoconvertible Protein SAASoti for the Study of Enzyme Activity in a Single Live Cell by Fluorescence Correlation Microscopy
- PMID: 35888428
- PMCID: PMC9316514
- DOI: 10.3390/ma15144962
Application of Genetically Encoded Photoconvertible Protein SAASoti for the Study of Enzyme Activity in a Single Live Cell by Fluorescence Correlation Microscopy
Abstract
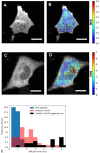
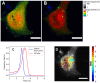
Fluorescent Correlation Spectroscopy (FCS) allows us to determine interactions of labeled proteins or changes in the oligomeric state. The FCS method needs a low amount of fluorescent dye, near nanomolar concentrations. To control the amount of fluorescent dye, we used new photoconvertible FP SAASoti. This work is devoted to the proof of principle of using photoconvertible proteins to measure caspase enzymatic activity in a single live cell. The advantage of this approach is that partial photoconversion of the FP makes FCS measurements possible when studying enzymatic reactions. To investigate the process, in vivo we used HeLa cell line expressing the engineered FRET sensor, SAASoti-23-KFP. This FRET sensor has a cleavable (DEVD) sequence in the linker between two FPs for the detection of one of the key enzymes of apoptosis, caspase-3. Caspase-3 activity was detected by registering the increase in the fluorescent lifetimes of the sensor, whereas the diffusion coefficient of SAASoti decreased. This can be explained by an increase in the total cell viscosity during apoptosis. We can suppose that in the moment of detectible caspase-3 activity, cell structure already has crucial changes in viscosity.
Keywords: FCS; FLIM; FRET sensor; caspase; photoconvertible FP; single cell.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

