Muscle Nutritive Metabolism Changes after Dietary Fishmeal Replaced by Cottonseed Meal in Golden Pompano (Trachinotus ovatus)
- PMID: 35888699
- PMCID: PMC9315803
- DOI: 10.3390/metabo12070576
Muscle Nutritive Metabolism Changes after Dietary Fishmeal Replaced by Cottonseed Meal in Golden Pompano (Trachinotus ovatus)
Abstract
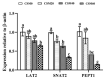
Our previous study demonstrated that based on growth performance and feed utilization, cottonseed meal (CSM) could substitute 20% fishmeal (FM) without adverse effect on golden pompano (Trachinotus ovatus). Muscle deposition was also an important indicator to evaluate the efficiency of alternative protein sources. Therefore, the present study was conducted to explore the changes of physiobiochemical and nutrient metabolism in muscle after FM replaced by CSM. Four isonitrogenous and isolipidic experimental diets (42.5% crude protein, 14.0% crude lipid) were formulated to replace 0% (CSM0 diet), 20% (CSM20 diet), 40% (CSM40 diet), and 60% (CSM60 diet) of FM with CSM. Juvenile fish (24.8 ± 0.02 g) were fed each diet for 6 weeks. The results presented, which, compared with the CSM0 diet, CSM20 and CSM40 diets, had no effect on changing the muscle proximate composition and free essential amino acid (EAA) concentration. For glycolipid metabolism, the CSM20 diet did not change the mRNA expression of hexokinase (hk), glucose transport protein 4 (glut4), glucagon-like peptide 1 receptor (glp-1r), while over 20% replacement impaired glucose metabolism. However, CSM20 and CSM40 diets had no effect on altering lipid metabolism. Mechanistically, compared with the CSM0 diet, the CSM20 diet did not change muscle nutritive metabolism through keeping the activities of the nutrient sensing signaling pathways stable. Higher replacement would break this balance and lead to muscle nutritive metabolism disorders. Based on the results, CSM could substitute 20-40% FM without affecting the muscle nutritive deposition. All data supplemented the powerful support for our previous conclusion that CSM could successfully replace 20% FM based on growth performance.
Keywords: cottonseed meal; fish nutrition; marine aquaculture; nutrient; physiobiochemical; replacement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

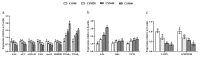
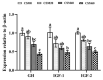

References
-
- Periago M.J., Ayala M.D., López-Albors O., Abdel I., Martínez C., García-Alcázar A., Ros G., Gil F. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture. 2005;249:175–188. doi: 10.1016/j.aquaculture.2005.02.047. - DOI
-
- Nemova N.N., Kantserova N.P., Lysenko L.A. The traits of protein metabolism in the skeletal muscle of teleost fish. J. Evol. Biochem. Physiol. 2021;57:626–645. doi: 10.1134/S0022093021030121. - DOI
-
- Willora F.P., Nadanasabesan N., Knutsen H.R., Liu C., Sørensen M., Hagen Ø. Growth performance, fast muscle development and chemical composition of juvenile lumpfish (Cyclopterus lumpus) fed diets incorporating soy and pea protein concentrates. Aquac. Rep. 2020;17:100352. doi: 10.1016/j.aqrep.2020.100352. - DOI
-
- Chen Y.K., Chi S.Y., Zhang S., Dong X.H., Yang Q.H., Liu H.Y., Tan B.P., Xie S.W. Evaluation of Methanotroph (Methylococcus capsulatus, Bath) bacteria meal on body composition, lipid metabolism, protein synthesis and muscle metabolites of Pacific white shrimp (Litopenaeus vannamei) Aquaculture. 2022;547:737517. doi: 10.1016/j.aquaculture.2021.737517. - DOI
-
- Quillet E., Guillou S.L., Aubin J., Labbé L., Fauconneau B., Médale F. Response of a lean muscle and a fat muscle rainbow trout (Oncorhynchus mykiss) line on growth, nutrient utilization, body composition and carcass traits when fed two different diets. Aquaculture. 2007;269:220–231. doi: 10.1016/j.aquaculture.2007.02.047. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

