A Comprehensive Study to Identify Major Metabolites of an Amoxicillin-Sulbactam Hybrid Molecule in Rats and Its Metabolic Pathway Using UPLC-Q-TOF-MS/MS
- PMID: 35888786
- PMCID: PMC9319383
- DOI: 10.3390/metabo12070662
A Comprehensive Study to Identify Major Metabolites of an Amoxicillin-Sulbactam Hybrid Molecule in Rats and Its Metabolic Pathway Using UPLC-Q-TOF-MS/MS
Abstract
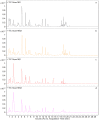
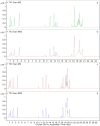
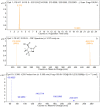
Amoxicillin and sulbactam are widely used compound drugs in animal food. The amoxicillin-sulbactam hybrid molecule can achieve better curative effects through the combination of the two drugs. However, its pharmacokinetic behavior needs to be explored. In this study, a randomized crossover experiment was performed to investigate the metabolism of the novel amoxicillin-sulbactam hybrid molecule in rats after gastric administration. Ultrahigh performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF-MS/MS) was used to isolate and to identify the metabolites in rats. Amoxicillin, amoxicilloic acid, amoxicillin diketopiperazine, and sulbactam were eventually detected in the plasma, liver, urine, and kidneys; no hybrid molecules and their metabolites were detected in feces. The in vivo metabolism results showed that the hybrid molecule was absorbed into the body in the intestine, producing amoxicillin and sulbactam, then amoxicillin was partially metabolized to amoxicilloic acid and amoxicillin diketopiperazine, which are eventually excreted in the urine by the kidneys. In this study, four major metabolites of the amoxicillin-sulbactam hybrid molecule were identified and their metabolic pathways were speculated, which provided scientific data for understanding the metabolism of the hybrid molecule and for its clinical rational use.
Keywords: amoxicillin; drug metabolism; hybrid molecule; sulbactam; ultrahigh performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


















References
-
- Mendonça S., Ecclissato C., Sartori M.S., Godoy A.P., Guerzoni R.A., Degger M., Pedrazzoli J., Jr. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxicillin, tetracycline, and furazolidone in Brazil. Helicobacter. 2000;5:79–83. doi: 10.1046/j.1523-5378.2000.00011.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

