A Comparative Study on Optofluidic Fenton Microreactors Integrated with Fe-Based Materials for Water Treatment
- PMID: 35888942
- PMCID: PMC9317202
- DOI: 10.3390/mi13071125
A Comparative Study on Optofluidic Fenton Microreactors Integrated with Fe-Based Materials for Water Treatment
Abstract
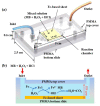
The catalysts employed in catalytic reactors greatly affect the reaction efficiency of the reaction system and the reactor’s performance. This work presents a rapid comparative study on three kinds of Fe-based materials integrated into an optofluidic Fenton reactor for water treatment. The Fe-based sheets (FeSiB, FeNbCuSiB, and FeNi) were respectively implanted into the reaction chamber to degrade the organic dyes with the assistance of H2O2. In the experiment, by adjusting the hydrogen peroxide concentration, flow rate, and light irradiation, the applicable conditions of the Fe-based materials for the dye degradation could be evaluated quickly to explore the optimal design of the Fenton reaction system. The results indicated that FeNi (1j85) exhibits excellent degradability in the microreactor, the reaction rate can reach 23.4%/s at the flow rate of 330 μL/min, but its weak corrosion resistance was definitely demonstrated. Although the initial degradability of the microreactor by using FeNbCuSiB (1k107) was not as good as that of 1j85, it increased after being reused several times instead, and the degradation efficiency reached >98% after being reused five times. However, the FeSiB (1k101) material shows the worst degradability and recycling. Therefore, in contrast, 1k107 has the greatest potential to be used in Fenton reactors for practical water treatment.
Keywords: Fe-based sheet; Fenton reaction; MB degradation; microreactor; reusability.
Conflict of interest statement
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Figures






Similar articles
-
Photo-Fenton-like degradation of antibiotics by inverse opal WO3 co-catalytic Fe2+/PMS, Fe2+/H2O2 and Fe2+/PDS processes: A comparative study.Chemosphere. 2022 Feb;288(Pt 3):132627. doi: 10.1016/j.chemosphere.2021.132627. Epub 2021 Oct 19. Chemosphere. 2022. PMID: 34678345
-
Fe-Immobilised Catechol-Based Hypercrosslinked Polymer as Heterogeneous Fenton Catalyst for Degradation of Methylene Blue in Water.Polymers (Basel). 2022 Jul 5;14(13):2749. doi: 10.3390/polym14132749. Polymers (Basel). 2022. PMID: 35808793 Free PMC article.
-
EDTA-Fe(III) Fenton-like oxidation for the degradation of malachite green.J Environ Manage. 2018 Nov 15;226:256-263. doi: 10.1016/j.jenvman.2018.08.029. Epub 2018 Aug 16. J Environ Manage. 2018. PMID: 30121461
-
A critical review on applications of microreactors for the treatment of polluted water with organic dyes.Sci Total Environ. 2025 Mar 15;969:178897. doi: 10.1016/j.scitotenv.2025.178897. Epub 2025 Feb 27. Sci Total Environ. 2025. PMID: 40010255 Review.
-
Hydroxylamine driven advanced oxidation processes for water treatment: A review.Chemosphere. 2021 Jan;262:128390. doi: 10.1016/j.chemosphere.2020.128390. Epub 2020 Sep 21. Chemosphere. 2021. PMID: 33182154 Review.
References
-
- Jin J., Dong L., Zhang K.H., Liu J. Recent Advances in Microfluidic Synthesis. Chin. J. Org. Chem. 2012;32:201–209. doi: 10.6023/cjoc1105131. - DOI
-
- Zhang X.D., Ma S., Li A.K., Chen L.Y., Lu J.W., Geng X.M., Xie M., Liang X.Y., Wan Y.F., Yang P. Continuous high-flux synthesis of gold nanoparticles with controllable sizes: A simple microfluidic system. Appl. Nanosci. 2020;10:661–669. doi: 10.1007/s13204-019-01214-y. - DOI
-
- Basheer C., Vetrichelvaw M., Perera A.P.P., Valiyaveettil S., Lee H.K. Oxidation of cyclohexene in a simple capellary-microreactor. Int. J. Nanosci. 2005;4:599–606. doi: 10.1142/S0219581X05003310. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

