Droplet Microfluidic Device for Chemoenzymatic Sensing
- PMID: 35888963
- PMCID: PMC9325247
- DOI: 10.3390/mi13071146
Droplet Microfluidic Device for Chemoenzymatic Sensing
Abstract
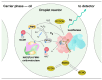
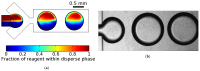
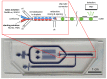
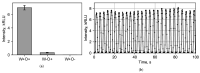
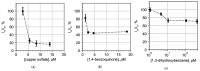
The rapid detection of pollutants in water can be performed with enzymatic probes, the catalytic light-emitting activity of which decreases in the presence of many types of pollutants. Herein, we present a microfluidic system for continuous chemoenzymatic biosensing that generates emulsion droplets containing two enzymes of the bacterial bioluminescent system (luciferase and NAD(P)H:FMN-oxidoreductase) with substrates required for the reaction. The developed chip generates "water-in-oil" emulsion droplets with a volume of 0.1 μL and a frequency of up to 12 drops per minute as well as provides the efficient mixing of reagents in droplets and their distancing. The bioluminescent signal from each individual droplet was measured by a photomultiplier tube with a signal-to-noise ratio of up to 3000/1. The intensity of the luminescence depended on the concentration of the copper sulfate with the limit of its detection of 5 μM. It was shown that bioluminescent enzymatic reactions could be carried out in droplet reactors in dispersed streams. The parameters and limitations required for the bioluminescent reaction to proceed were also studied. Hereby, chemoenzymatic sensing capabilities powered by a droplet microfluidics manipulation technique may serve as the basis for early-warning online water pollution systems.
Keywords: bioluminescence; chemoenzymatic system; droplet microfluidics; luciferase; sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Roy S.P. Overview of heavy metals and aquatic environment with notes on their recovery. Ecoscan. 2010;4:235–240.
-
- Gumpu M.B., Sethuraman S., Krishnan U.M., Rayappan J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015;213:515–533. doi: 10.1016/j.snb.2015.02.122. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

