Immunosuppressive Mechanisms in Brucellosis in Light of Chronic Bacterial Diseases
- PMID: 35888979
- PMCID: PMC9324529
- DOI: 10.3390/microorganisms10071260
Immunosuppressive Mechanisms in Brucellosis in Light of Chronic Bacterial Diseases
Abstract
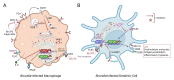
Brucellosis is considered one of the major zoonoses worldwide, constituting a critical livestock and human health concern with a huge socio-economic burden. Brucella genus, its etiologic agent, is composed of intracellular bacteria that have evolved a prodigious ability to elude and shape host immunity to establish chronic infection. Brucella's intracellular lifestyle and pathogen-associated molecular patterns, such as its specific lipopolysaccharide (LPS), are key factors for hiding and hampering recognition by the immune system. Here, we will review the current knowledge of evading and immunosuppressive mechanisms elicited by Brucella species to persist stealthily in their hosts, such as those triggered by their LPS and cyclic β-1,2-d-glucan or involved in neutrophil and monocyte avoidance, antigen presentation impairment, the modulation of T cell responses and immunometabolism. Attractive strategies exploited by other successful chronic pathogenic bacteria, including Mycobacteria, Salmonella, and Chlamydia, will be also discussed, with a special emphasis on the mechanisms operating in brucellosis, such as granuloma formation, pyroptosis, and manipulation of type I and III IFNs, B cells, innate lymphoid cells, and host lipids. A better understanding of these stratagems is essential to fighting bacterial chronic infections and designing innovative treatments and vaccines.
Keywords: Brucella; chronic infection; immunosuppression; intracellular bacteria; persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Establishment of Chronic Infection: Brucella's Stealth Strategy.Front Cell Infect Microbiol. 2016 Mar 15;6:30. doi: 10.3389/fcimb.2016.00030. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27014640 Free PMC article. Review.
-
Transcriptome analysis of macrophages during Brucella abortus infection clarifies the survival mechanisms of the bacteria.Diagn Microbiol Infect Dis. 2024 Sep;110(1):116401. doi: 10.1016/j.diagmicrobio.2024.116401. Epub 2024 Jun 12. Diagn Microbiol Infect Dis. 2024. PMID: 38878343
-
Immunometabolism in human brucellosis: An emerging field of investigation.Microb Pathog. 2021 Sep;158:105115. doi: 10.1016/j.micpath.2021.105115. Epub 2021 Jul 28. Microb Pathog. 2021. PMID: 34332069 Review.
-
Development and evaluation of the Galleria mellonella (greater wax moth) infection model to study Brucella host-pathogen interaction.Microb Pathog. 2023 Jan;174:105930. doi: 10.1016/j.micpath.2022.105930. Epub 2022 Dec 7. Microb Pathog. 2023. PMID: 36496059
-
Recent developments in livestock and wildlife brucellosis vaccination.Rev Sci Tech. 2013 Apr;32(1):207-17. doi: 10.20506/rst.32.1.2201. Rev Sci Tech. 2013. PMID: 23837378 Review.
Cited by
-
Beyond its preferential niche: Brucella abortus RNA down-modulates the IFN-γ-induced MHC-I expression in epithelial and endothelial cells.PLoS One. 2024 Jul 9;19(7):e0306429. doi: 10.1371/journal.pone.0306429. eCollection 2024. PLoS One. 2024. PMID: 38980867 Free PMC article.
-
Clinical characterization of brucellosis in children from non-pastoral areas: a report of five cases.BMC Infect Dis. 2024 Sep 8;24(1):929. doi: 10.1186/s12879-024-09843-7. BMC Infect Dis. 2024. PMID: 39245722 Free PMC article.
-
SLAMF7 and SLAMF8 receptors shape human plasmacytoid dendritic cell responses to intracellular bacteria.J Clin Invest. 2025 Apr 15;135(8):e182467. doi: 10.1172/JCI182467. eCollection 2025 Apr 15. J Clin Invest. 2025. PMID: 40231463 Free PMC article.
-
Doxycycline-Induced Apoptosis in Brucella suis S2-Infected HMC3 Cells via Calreticulin Suppression and Activation of the IRE1/Caspase-3 Signaling Pathway.Infect Drug Resist. 2025 Apr 23;18:2005-2020. doi: 10.2147/IDR.S507193. eCollection 2025. Infect Drug Resist. 2025. PMID: 40290405 Free PMC article.
-
Brucella abortus impairs T lymphocyte responsiveness by mobilizing IL-1RA-secreting omental neutrophils.Nat Commun. 2025 Jan 20;16(1):862. doi: 10.1038/s41467-024-55799-2. Nat Commun. 2025. PMID: 39833171 Free PMC article.
References
-
- Moreno E., Moriyón I. The Genus Brucella. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., editors. The Prokaryotes: Volume 5: Proteobacteria: Alpha and Beta Subclasses. Springer; New York, NY, USA: 2006. pp. 315–456.
Publication types
LinkOut - more resources
Full Text Sources

