Human Breast Milk: A Source of Potential Probiotic Candidates
- PMID: 35888998
- PMCID: PMC9319366
- DOI: 10.3390/microorganisms10071279
Human Breast Milk: A Source of Potential Probiotic Candidates
Abstract
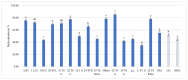
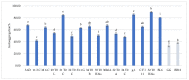
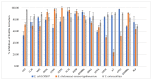
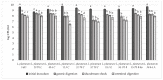
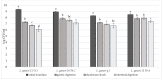
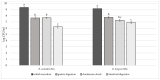
This study focuses on the isolation of lactobacilli/bifidobacteria from human breast milk and their first characterization, in the perspective to find new probiotic candidates to be included in food products. More specifically, breast-milk-isolated strains demonstrated a very good aptitude to adhere to intestinal cells, in comparison with L. rhamnosus GG strain, taken as reference. The same behavior has been found for hydrophobicity/auto-aggregation properties. A remarkable antagonistic activity was detected for these isolates not only against spoilage and pathogenic species of food interest, but also against the principal etiological agents of intestinal infections. Indeed, isolated strains impaired spoilage and pathogenic species growth, as well as biofilm formation by gut pathogens. In addition, breast milk strains were characterized for their antibiotic susceptibility, displaying species-specific and strain-specific susceptibility patterns. Finally, to assess their technological potential, the fermentation kinetics and viability of breast milk strains in pasteurized milk were investigated, also including the study of the volatile molecule profiles. In this regard, all the strains pointed out the release of aroma compounds frequently associated with the sensory quality of several dairy products such as acetic acid, diacetyl, acetoin, acetaldehyde. Data here reported point up the high potential of breast-milk-isolated strains as probiotics.
Keywords: bifidobacteria; breast milk; lactobacilli; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

