Seed Protection of Solanum lycopersicum with Pythium oligandrum against Alternaria brassicicola and Verticillium albo-atrum
- PMID: 35889067
- PMCID: PMC9315653
- DOI: 10.3390/microorganisms10071348
Seed Protection of Solanum lycopersicum with Pythium oligandrum against Alternaria brassicicola and Verticillium albo-atrum
Abstract
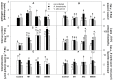
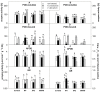
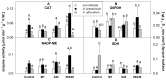
Pythium oligandrum, strain M1, is a soil oomycete successfully used as a biological control agent (BCA), protecting plants against fungal, yeast, and oomycete pathogens through mycoparasitism and elicitor-dependent plant priming. The not yet described Pythium strains, X42 and 00X48, have shown potential as BCAs given the high activity of their secreted proteases, endoglycosidases, and tryptamine. Here, Solanum lycopersicum L. cv. Micro-Tom seeds were coated with Pythium strains, and seedlings were exposed to fungal pathogens, either Alternaria brassicicola or Verticillium albo-atrum. The effects of both infection and seed-coating on plant metabolism were assessed by determining the activity and isoforms of antioxidant enzymes and endoglycosidases and the content of tryptamine, amino acids, and heat shock proteins. Dual culture competition testing and microscopy analysis confirmed mycoparasitism in all three Pythium strains. In turn, seed treatment significantly increased the total free amino acid content, changing their abundance in both non-infected and infected plants. In response to pathogens, plant Hsp70 and Hsp90 isoform levels also varied among Pythium strains, most likely as a strategy for priming the plant against infection. Overall, our results show in vitro mycoparasitism between Pythium strains and fungal pathogens and in planta involvement of heat shock proteins in priming.
Keywords: antioxidants; capillary electrophoresis; fungal diseases; plant protection; seed-coating.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Barrit T., Porcher A., Cukier C., Satour P., Guillemette T., Limami A.M., Teulat B., Campion C., Planchet E. Nitrogen nutrition modifies the susceptibility of Arabidopsis thaliana to the necrotrophic fungus, Alternaria brassicicola. Physiol. Plant. 2021;174:e13621. doi: 10.1111/ppl.13621. - DOI - PubMed
-
- Tralamazza S.M., Piacentini K.C., Iwase C.H.T., Rocha L.D. Toxigenic Alternaria species: Impact in cereals worldwide. Curr. Opin. Food Sci. 2018;23:57–63. doi: 10.1016/j.cofs.2018.05.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

