Characterization and Comparison of Ocular Surface Microbiome in Newborns
- PMID: 35889110
- PMCID: PMC9320102
- DOI: 10.3390/microorganisms10071390
Characterization and Comparison of Ocular Surface Microbiome in Newborns
Abstract
The ocular microbiome is of fundamental importance for immune eye homeostasis, and its alteration would lead to an impairment of ocular functionality. Little evidence is reported on the composition of the ocular microbiota of term infants and on the impact of antibiotic prophylaxis.
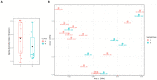
Methods: A total of 20 conjunctival swabs were collected from newborns at birth and after antibiotic treatment. Samples were subjected to 16S rRNA sequencing via system MiSeq Illumina. The data were processed with the MicrobAT software and statistical analysis were performed using two-way ANOVA.
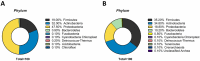
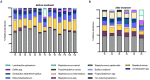
Results: Antibiotic prophylaxis with gentamicin altered the composition of the microbiota. In detail, a 1.5- and 2.01-fold reduction was recorded for Cutibacterium acnes (C. acnes) and Massilia timonae (M. timonae), respectively, whereas an increase in Staphylococcus spp. of 6.5 times occurred after antibiotic exposure.
Conclusions: Antibiotic prophylaxis altered the ocular microbiota whose understanding could avoid adverse effects on eye health.
Keywords: 16S rRNA sequencing; bacteria; newborn; ocular surface microbiota.
Conflict of interest statement
Lara M. V. Boatti has been involved as a consultant and expert witness in Arrow Diagnostics.
Figures

Similar articles
-
Cutibacterium acnes (Propionibacterium acnes) 16S rRNA Genotyping of Microbial Samples from Possessions Contributes to Owner Identification.mSystems. 2019 Nov 26;4(6):e00594-19. doi: 10.1128/mSystems.00594-19. mSystems. 2019. PMID: 31771975 Free PMC article.
-
Perinatal Antibiotic Exposure Affects the Transmission between Maternal and Neonatal Microbiota and Is Associated with Early-Onset Sepsis.mSphere. 2020 Feb 19;5(1):e00984-19. doi: 10.1128/mSphere.00984-19. mSphere. 2020. PMID: 32075882 Free PMC article.
-
Initial description of the core ocular surface microbiome in dogs: Bacterial community diversity and composition in a defined canine population.Vet Ophthalmol. 2019 May;22(3):337-344. doi: 10.1111/vop.12599. Epub 2018 Aug 10. Vet Ophthalmol. 2019. PMID: 30095241
-
The Ocular Microbiome: Molecular Characterisation of a Unique and Low Microbial Environment.Curr Eye Res. 2019 Jul;44(7):685-694. doi: 10.1080/02713683.2019.1570526. Epub 2019 Feb 4. Curr Eye Res. 2019. PMID: 30640553 Review.
-
Current knowledge on the human eye microbiome: a systematic review of available amplicon and metagenomic sequencing data.Acta Ophthalmol. 2021 Feb;99(1):16-25. doi: 10.1111/aos.14508. Epub 2020 Jun 29. Acta Ophthalmol. 2021. PMID: 32602257
Cited by
-
Dual Role of Cutibacterium acnes: Commensal Bacterium and Pathogen in Ocular Diseases.Microorganisms. 2024 Aug 12;12(8):1649. doi: 10.3390/microorganisms12081649. Microorganisms. 2024. PMID: 39203490 Free PMC article.
-
Paediatric Utilisation of Ophthalmic Antibiotics in the Ear in Aotearoa/New Zealand.Children (Basel). 2025 Apr 25;12(5):557. doi: 10.3390/children12050557. Children (Basel). 2025. PMID: 40426736 Free PMC article.
-
Broad-Spectrum Antimicrobial Activity of Oftasecur and Visuprime Ophthalmic Solutions.Microorganisms. 2023 Feb 17;11(2):503. doi: 10.3390/microorganisms11020503. Microorganisms. 2023. PMID: 36838468 Free PMC article.
-
Characterization of Gut Microbiota Composition in Type 2 Diabetes Patients: A Population-Based Study.Int J Environ Res Public Health. 2022 Nov 29;19(23):15913. doi: 10.3390/ijerph192315913. Int J Environ Res Public Health. 2022. PMID: 36497987 Free PMC article.
-
Atopic Dermatitis and Atopic Keratoconjunctivitis: New Insights in the Analyses of Microbiota and Probiotic Effect.Int J Mol Sci. 2025 Feb 10;26(4):1463. doi: 10.3390/ijms26041463. Int J Mol Sci. 2025. PMID: 40003928 Free PMC article. Review.
References
-
- Dell’Annunziata F., Dell’Aversana C., Doti N., Donadio G., Dal Piaz F., Izzo V., De Filippis A., Galdiero M., Altucci L., Boccia G., et al. Outer Membrane Vesicles Derived from Klebsiella Pneumoniae Are a Driving Force for Horizontal Gene Transfer. Int. J. Mol. Sci. 2021;22:8732. doi: 10.3390/ijms22168732. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

