Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging-A Review
- PMID: 35889124
- PMCID: PMC9320618
- DOI: 10.3390/microorganisms10071405
Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging-A Review
Abstract
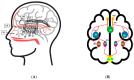
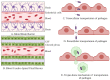
The nasal region is one of the distinct environments for the survival of various microbiota. The human microbial niche begins to inhabit the human body right from birth, and the microbiota survive as commensals or opportunistic pathogens throughout the life of humans in their bodies in various habitats. These microbial communities help to maintain a healthy microenvironment by preventing the attack of pathogens and being involved in immune regulation. Any dysbiosis of microbiota residing in the mucosal surfaces, such as the nasal passages, guts, and genital regions, causes immune modulation and severe infections. The coexistence of microorganisms in the mucosal layers of respiratory passage, resulting in infections due to their co-abundance and interactions, and the background molecular mechanisms responsible for such interactions, need to be considered for investigation. Additional clinical evaluations can explain the interactions among the nasal microbiota, nasal dysbiosis and neurodegenerative diseases (NDs). The respiratory airways usually act as a substratum place for the microbes and can act as the base for respiratory tract infections. The microbial metabolites and the microbes can cross the blood-brain barrier and may cause NDs, such as Parkinson's disease (PD), Alzheimer's disease (AD), and multiple sclerosis (MS). The scientific investigations on the potential role of the nasal microbiota in olfactory functions and the relationship between their dysfunction and neurological diseases are limited. Recently, the consequences of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) in patients with neurological diseases are under exploration. The crosstalk between the gut and the nasal microbiota is highly influential, because their mucosal regions are the prominent microbial niche and are connected to the olfaction, immune regulation, and homeostasis of the central nervous system. Diet is one of the major factors, which strongly influences the mucosal membranes of the airways, gut, and lung. Unhealthy diet practices cause dysbiosis in gut microbiota and the mucosal barrier. The current review summarizes the interrelationship between the nasal microbiota dysbiosis, resulting olfactory dysfunctions, and the progression of NDs during aging and the involvement of coronavirus disease 2019 in provoking the NDs.
Keywords: Alzheimer’s disease; COVID-19; Parkinson’s disease; SARS-CoV-2; multiple sclerosis; nasal microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

