A Chikungunya Virus Multiepitope Recombinant Protein Expressed from the Binary System Insect Cell/Recombinant Baculovirus Is Useful for Laboratorial Diagnosis of Chikungunya
- PMID: 35889170
- PMCID: PMC9316945
- DOI: 10.3390/microorganisms10071451
A Chikungunya Virus Multiepitope Recombinant Protein Expressed from the Binary System Insect Cell/Recombinant Baculovirus Is Useful for Laboratorial Diagnosis of Chikungunya
Abstract
Chikungunya virus (CHIKV) is an arbovirus currently distributed worldwide, causing a disease that shares clinical signs and symptoms with other illnesses, such as dengue and Zika and leading to a challenging clinical differential diagnosis. In Brazil, CHIKV emerged in 2014 with the simultaneous introduction of both Asian and East/Central/South African (ECSA) genotypes. Laboratorial diagnosis of CHIKV is mainly performed by molecular and serological assays, with the latter more widely used. Although many commercial kits are available, their costs are still high for many underdeveloped and developing countries where the virus circulates. Here we described the development and evaluation of a multi-epitope recombinant protein-based IgG-ELISA (MULTREC IgG-ELISA) test for the specific detection of anti-CHIKV antibodies in clinical samples, as an alternative approach for laboratorial diagnosis. The MULTREC IgG-ELISA showed 86.36% of sensitivity and 100% of specificity, and no cross-reactivity with other exanthematic diseases was observed. The recombinant protein was expressed from the binary system insect cell/baculovirus using the crystal-forming baculoviral protein polyhedrin as a carrier of the target recombinant protein to facilitate recovery. The crystals were at least 10 times smaller in size and had an amorphous shape when compared to the polyhedrin wild-type crystal. The assay uses a multi-epitope antigen, representing two replicates of 18 amino acid sequences from the E2 region and a sequence of 17 amino acids from the nsP3 region of CHIKV. The recombinant protein was highly expressed, easy to purify and has demonstrated its usefulness in confirming chikungunya exposure, indeed showing a good potential tool for epidemiological surveillance.
Keywords: E2; IgG-ELISA; baculovirus expression; chikungunya; recombinant protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


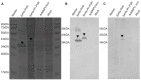
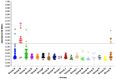
References
-
- Chikungunya Cases Identified Through Passive Surveillance and Household Investigations—Puerto Rico, May 5–August 12, 2014. [(accessed on 2 December 2021)]; Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6348a1.htm. - PMC - PubMed
Grants and funding
- E-26/202.003/2016/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/202.659/2019/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/211.569/2019/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- 302462/2018-0/National Council for Scientific and Technological Development
- 305468/2019-7/National Council for Scientific and Technological Development
LinkOut - more resources
Full Text Sources

