Doxorubicin-Based Hybrid Compounds as Potential Anticancer Agents: A Review
- PMID: 35889350
- PMCID: PMC9318127
- DOI: 10.3390/molecules27144478
Doxorubicin-Based Hybrid Compounds as Potential Anticancer Agents: A Review
Abstract
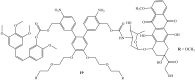
The scarcity of novel and effective therapeutics for the treatment of cancer is a pressing and alarming issue that needs to be prioritized. The number of cancer cases and deaths are increasing at a rapid rate worldwide. Doxorubicin, an anticancer agent, is currently used to treat several types of cancer. It disrupts myriad processes such as histone eviction, ceramide overproduction, DNA-adduct formation, reactive oxygen species generation, Ca2+, and iron hemostasis regulation. However, its use is limited by factors such as drug resistance, toxicity, and congestive heart failure reported in some patients. The combination of doxorubicin with other chemotherapeutic agents has been reported as an effective treatment option for cancer with few side effects. Thus, the hybridization of doxorubicin and other chemotherapeutic drugs is regarded as a promising approach that can lead to effective anticancer agents. This review gives an update on hybrid compounds containing the scaffolds of doxorubicin and its derivatives with potent chemotherapeutic effects.
Keywords: cancer; chemotherapeutic drugs; doxorubicin; drug resistance; drug toxicity; hybrid compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

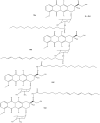
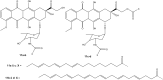
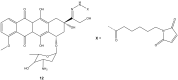

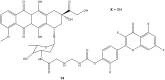

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

