Kinetic Characteristics of Curcumin and Germacrone in Rat and Human Liver Microsomes: Involvement of CYP Enzymes
- PMID: 35889364
- PMCID: PMC9317718
- DOI: 10.3390/molecules27144482
Kinetic Characteristics of Curcumin and Germacrone in Rat and Human Liver Microsomes: Involvement of CYP Enzymes
Abstract
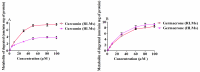
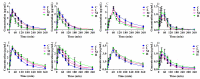
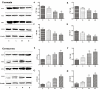
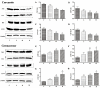
Curcumin and germacrone, natural products present in the Zingiberaceae family of plants, have several biological properties. Among these properties, the anti-NSCLC cancer action is noteworthy. In this paper, kinetics of the two compounds in rat liver microsomes (RLMs), human liver microsomes (HLMs), and cytochrome P450 (CYP) enzymes (CYP3A4, 1A2, 2E1, and 2C19) in an NADPH-generating system in vitro were evaluated by UP-HPLC-MS/MS (ultrahigh-pressure liquid chromatography-tandem mass spectrometry). The contents of four cytochrome P450 (CYP) enzymes, adjusting by the compounds were detected using Western blotting in vitro and in vivo. The t1/2 of curcumin was 22.35 min in RLMs and 173.28 min in HLMs, while 18.02 and 16.37 min were gained for germacrone. The Vmax of curcumin in RLMs was about 4-fold in HLMs, meanwhile, the Vmax of germacrone in RLMs was similar to that of HLMs. The single enzyme t1/2 of curcumin was 38.51 min in CYP3A4, 301.4 min in 1A2, 69.31 min in 2E1, 63.01 min in 2C19; besides, as to the same enzymes, t1/2 of germacrone was 36.48 min, 86.64 min, 69.31 min, and 57.76 min. The dynamic curves were obtained by reasonable experimental design and the metabolism of curcumin and germacrone were selected in RLMs/HLMs. The selectivities in the two liver microsomes differed in degradation performance. These results meant that we should pay more attention to drugs in clinical medication-drug and drug-enzyme interactions.
Keywords: UPLC–MS/MS; curcumin; cytochrome P450 enzymes; drug–drug interaction; germacrone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

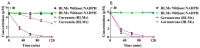


Similar articles
-
Interactions of sesquiterpenes zederone and germacrone with the human cytochrome P450 system.Toxicol In Vitro. 2013 Sep;27(6):2005-12. doi: 10.1016/j.tiv.2013.07.004. Epub 2013 Jul 12. Toxicol In Vitro. 2013. PMID: 23850985
-
Comparative metabolism of tussilagone in rat and human liver microsomes using ultra-high-performance liquid chromatography coupled with high-resolution LTQ-Orbitrap mass spectrometry.Rapid Commun Mass Spectrom. 2015 Sep 30;29(18):1641-50. doi: 10.1002/rcm.7262. Rapid Commun Mass Spectrom. 2015. PMID: 26467116
-
Inhibitory effects of the main metabolites of Apatinib on CYP450 isozymes in human and rat liver microsomes.Toxicol In Vitro. 2024 Mar;95:105739. doi: 10.1016/j.tiv.2023.105739. Epub 2023 Dec 1. Toxicol In Vitro. 2024. PMID: 38042355
-
Roles of cytochrome P450 3A enzymes in the 2-hydroxylation of 1,4-cineole, a monoterpene cyclic ether, by rat and human liver microsomes.Xenobiotica. 2001 Oct;31(10):713-23. doi: 10.1080/00498250110065595. Xenobiotica. 2001. PMID: 11695850
-
Germacrone: A Multi-targeting Sesquiterpene with Promising Anti-cancer and Chronic Disease Applications.Anticancer Agents Med Chem. 2024;24(19):1396-1406. doi: 10.2174/0118715206312324240805075050. Anticancer Agents Med Chem. 2024. PMID: 39113300
References
-
- Luo S.H., Weng X.H., Lin S., Huang X.T., Huang L.N., Zhou W., Guo X.Z., Xu X.W. Evaluation of osimertinib for advanced non-small cell lung cancer with leptomeningeal metastases: A cost-effectiveness and budget impact analysis. Int. J. Clin. Pharm. 2022;44:192–200. doi: 10.1007/s11096-021-01333-z. - DOI - PubMed
-
- Oglah M.K., Mustafa Y.F. Curcumin analogs: Synthesis and biological activities. Med. Chem. Res. 2019;29:479–486. doi: 10.1007/s00044-019-02497-0. - DOI
-
- Namwan N., Senawong G., Phaosiri C., Kumboonma P., Somsakeesit L.O., Samankul A., Leerat C., Leerat T. HDAC inhibitory and anti-cancer activities of curcumin and curcumin derivative CU17 against human lung cancer A549 cells. Molecules. 2022;27:4014. doi: 10.3390/molecules27134014. - DOI - PMC - PubMed
-
- Wei Z.S., Niranjan A., Abou-Al-Shaar H., Deng H.S., Albano L., Lunsford L.D. A volume matched comparison of survival after radiosurgery in non-small cell lung cancer patients with one versus more than twenty brain metastases. J. Neuro-Oncol. 2022;157:417–423. doi: 10.1007/s11060-022-03981-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

