Advances in Fibrin-Based Materials in Wound Repair: A Review
- PMID: 35889381
- PMCID: PMC9322155
- DOI: 10.3390/molecules27144504
Advances in Fibrin-Based Materials in Wound Repair: A Review
Abstract
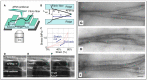
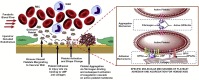
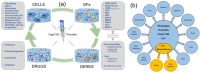
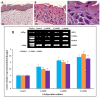
The first bioprocess that occurs in response to wounding is the deterrence of local hemorrhage. This is accomplished by platelet aggregation and initiation of the hemostasis cascade. The resulting blood clot immediately enables the cessation of bleeding and then functions as a provisional matrix for wound healing, which begins a few days after injury. Here, fibrinogen and fibrin fibers are the key players, because they literally serve as scaffolds for tissue regeneration and promote the migration of cells, as well as the ingrowth of tissues. Fibrin is also an important modulator of healing and a host defense system against microbes that effectively maintains incoming leukocytes and acts as reservoir for growth factors. This review presents recent advances in the understanding and applications of fibrin and fibrin-fiber-incorporated biomedical materials applied to wound healing and subsequent tissue repair. It also discusses how fibrin-based materials function through several wound healing stages including physical barrier formation, the entrapment of bacteria, drug and cell delivery, and eventual degradation. Pure fibrin is not mechanically strong and stable enough to act as a singular wound repair material. To alleviate this problem, this paper will demonstrate recent advances in the modification of fibrin with next-generation materials exhibiting enhanced stability and medical efficacy, along with a detailed look at the mechanical properties of fibrin and fibrin-laden materials. Specifically, fibrin-based nanocomposites and their role in wound repair, sustained drug release, cell delivery to wound sites, skin reconstruction, and biomedical applications of drug-loaded fibrin-based materials will be demonstrated and discussed.
Keywords: drug release; fibrin; fibrinogen; nanofibers; protein; wound healing.
Conflict of interest statement
The author declares no conflict of interest.
Figures






References
-
- Scott E.M., Ariëns R.A., Grant P.J. Genetic and environmental determinants of fibrin structure and function: Relevance to clinical disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:1558–1566. doi: 10.1161/01.ATV.0000136649.83297.bf. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

