Formulation of Chrysomycin A Cream for the Treatment of Skin Infections
- PMID: 35889485
- PMCID: PMC9323865
- DOI: 10.3390/molecules27144613
Formulation of Chrysomycin A Cream for the Treatment of Skin Infections
Abstract
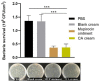
Chrysomycin A, a compound derived from marine microorganisms, proved to have a specific great in vitro inhibitory effect on methicillin-resistant Staphylococcus aureus (MRSA). It exhibits high safety for the skin, as well as a better therapeutic effect than the current clinical drug, vancomycin. Nevertheless, its poor water solubility highly limits the application and reduces the bioavailability. In view of this, we developed a cream of chrysomycin A (CA) to enhance the solubility for the treatment of skin infection, while avoiding the possible toxicity caused by systemic administration. A comprehensive orthogonal evaluation system composed of appearance, spreading ability, and stability was established to find the optimal formula under experimental conditions. The final product was odorless and easy to be spread, with a lustrous, smooth surface. The particle size of the product met Chinese Pharmacopoeia specifications and the entire cream showed long-term stability in destructive tests. The in vitro and in vivo studies indicated that CA cream had a similar anti-MRSA activity to commercially available mupirocin, showing its potential as an efficacious topical delivery system for skin infections treatment.
Keywords: MRSA; chrysomycin A; cream; skin infections; topical administration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jevons M.P. “Celbenin”-Resistant Staphylococcus. Br. Med. J. 1961;1:124–125. doi: 10.1136/bmj.1.5219.124-a. - DOI
-
- Boswihi S.S., Udo E.E. Methicillin-resistant Staphylococcus aureus: An update on the epidemiology, treatment options and infection control. Curr. Med. Res. Pract. 2018;8:18–24. doi: 10.1016/j.cmrp.2018.01.001. - DOI
-
- Chang L., Liu J. Antimicrobial treatment of Staphylococcus aureus infection in skin and soft tissue. Health Prot. Promot. 2016;16:60.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

