Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water
- PMID: 35889573
- PMCID: PMC9315505
- DOI: 10.3390/nano12142348
Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water
Abstract
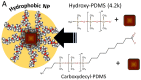
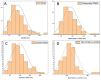
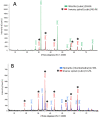
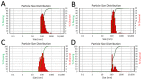
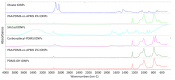
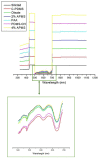
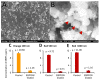
Nanoplastic pollution is increasing worldwide and poses a threat to humans, animals, and ecological systems. High-throughput, reliable methods for the isolation and separation of NMPs from drinking water, wastewater, or environmental bodies of water are of interest. We investigated iron oxide nanoparticles (IONPs) with hydrophobic coatings to magnetize plastic particulate waste for removal. We produced and tested IONPs synthesized using air-free conditions and in atmospheric air, coated with several polydimethylsiloxane (PDMS)-based hydrophobic coatings. Particles were characterized with scanning electron microscopy (SEM), transmission electron microscopy (TEM), superconducting quantum interference device (SQUID) magnetometry, dynamic light scattering (DLS), X-ray diffraction (XRD) and zeta potential. The IONPs synthesized in air contained a higher percentage of the magnetic spinel phase and stronger magnetization. Binding and recovery of NMPs from both salt and freshwater samples was demonstrated. Specifically, we were able to remove 100% of particles in a range of sizes, from 2-5 mm, and nearly 90% of nanoplastic particles with a size range from 100 nm to 1000 nm using a simple 2-inch permanent NdFeB magnet. Magnetization of NMPs using IONPs is a viable method for separation from water samples for quantification, characterization, and purification and remediation of water.
Keywords: PDMS; aminopropylsiloxane; amphiphilic polymer; hydrophobic coatings; hydrophobicity; interparticle interactions; iron oxide nanoparticles; microplastics; nanoplastics; plastic pollution; polydimethylsiloxane nanocomposite; separation science; water remediation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Eriksen M., Lebreton L.C., Carson H.S., Thiel M., Moore C.J., Borerro J.C., Galgani F., Ryan P.G., Reisser J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE. 2014;9:e111913. doi: 10.1371/journal.pone.0111913. - DOI - PMC - PubMed

