Stable Dried Catalase Particles Prepared by Electrospraying
- PMID: 35889708
- PMCID: PMC9322511
- DOI: 10.3390/nano12142484
Stable Dried Catalase Particles Prepared by Electrospraying
Abstract
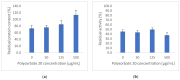
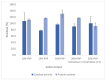
Therapeutic proteins and peptides are clinically important, offering potency while reducing the potential for off-target effects. Research interest in developing therapeutic polypeptides has grown significantly during the last four decades. However, despite the growing research effort, maintaining the stability of polypeptides throughout their life cycle remains a challenge. Electrohydrodynamic (EHD) techniques have been widely explored for encapsulation and delivery of many biopharmaceuticals. In this work, we explored monoaxial electrospraying for encapsulation of bovine liver catalase, investigating the effects of the different components of the electrospraying solution on the integrity and bioactivity of the enzyme. The catalase was successfully encapsulated within polymeric particles made of polyvinylpyrrolidone (PVP), dextran, and polysucrose. The polysorbate 20 content within the electrospraying solution (50 mM citrate buffer, pH 5.4) affected the catalase loading-increasing the polysorbate 20 concentration to 500 μg/mL resulted in full protein encapsulation but did not prevent loss in activity. The addition of ethanol (20% v/v) to a fully aqueous solution improves the electrospraying process by reducing surface tension, without loss of catalase activity. The polymer type was shown to have the greatest impact on preserving catalase activity within the electrosprayed particles. When PVP was the carrier there was no loss in activity compared with fresh aqueous solutions of catalase. The optimum particles were obtained from a 20% w/v PVP or 30% w/v PVP-trehalose (1:1 w/w) solution. The addition of trehalose confers stability advantages to the catalase particles. When trehalose-PVP particles were stored at 5 °C, enzymatic activity was maintained over 3 months, whereas for the PVP-only analogue a 50% reduction in activity was seen. This demonstrates that processing catalase by monoaxial electrospraying can, under optimised conditions, result in stable polymeric particles with no loss of activity.
Keywords: bovine liver catalase; electrospraying; protein stability; proteins; solid-state protein formulation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









References
-
- Crommelin D.J.A., Sindelar R.D., Meibohm B. Pharmaceutical Biotechnology: Fundamentals and Applications. 5th ed. Springer International Publishing; Cham, Switzerland: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources

