Biomimetic Nanomaterials: Diversity, Technology, and Biomedical Applications
- PMID: 35889709
- PMCID: PMC9316400
- DOI: 10.3390/nano12142485
Biomimetic Nanomaterials: Diversity, Technology, and Biomedical Applications
Abstract
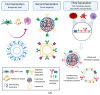
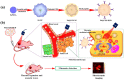
Biomimetic nanomaterials (BNMs) are functional materials containing nanoscale components and having structural and technological similarities to natural (biogenic) prototypes. Despite the fact that biomimetic approaches in materials technology have been used since the second half of the 20th century, BNMs are still at the forefront of materials science. This review considered a general classification of such nanomaterials according to the characteristic features of natural analogues that are reproduced in the preparation of BNMs, including biomimetic structure, biomimetic synthesis, and the inclusion of biogenic components. BNMs containing magnetic, metal, or metal oxide organic and ceramic structural elements (including their various combinations) were considered separately. The BNMs under consideration were analyzed according to the declared areas of application, which included tooth and bone reconstruction, magnetic and infrared hyperthermia, chemo- and immunotherapy, the development of new drugs for targeted therapy, antibacterial and anti-inflammatory therapy, and bioimaging. In conclusion, the authors' point of view is given about the prospects for the development of this scientific area associated with the use of native, genetically modified, or completely artificial phospholipid membranes, which allow combining the physicochemical and biological properties of biogenic prototypes with high biocompatibility, economic availability, and scalability of fully synthetic nanomaterials.
Keywords: applications; biomedicine; biomimetics; nanomaterials; nanoparticles; synthesis technique; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Das D., Noh I. Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting. Springer; Berlin/Heidelberg, Germany: 2018.
-
- Ruys A.J. Biomimetic Biomaterials. Elsevier; Amsterdam, The Netherlands: 2013.
-
- Poupon E., Nay B. Biomimetic Organic Synthesis. Volume 1–2. Wiley; Hoboken, NJ, USA: 2011.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

