β-Sitosterol Attenuates Dexamethasone-Induced Muscle Atrophy via Regulating FoxO1-Dependent Signaling in C2C12 Cell and Mice Model
- PMID: 35889851
- PMCID: PMC9315776
- DOI: 10.3390/nu14142894
β-Sitosterol Attenuates Dexamethasone-Induced Muscle Atrophy via Regulating FoxO1-Dependent Signaling in C2C12 Cell and Mice Model
Abstract
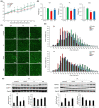
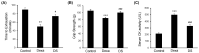
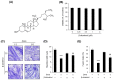
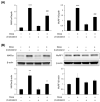
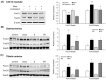
Sarcopenia refers to a decline in muscle mass and strength with age, causing significant impairment in the ability to carry out normal daily functions and increased risk of falls and fractures, eventually leading to loss of independence. Maintaining protein homeostasis is an important factor in preventing muscle loss, and the decrease in muscle mass is caused by an imbalance between anabolism and catabolism of muscle proteins. Although β-sitosterol has various effects such as anti-inflammatory, protective effect against nonalcoholic fatty liver disease (NAFLD), antioxidant, and antidiabetic activity, the mechanism of β-sitosterol effect on the catabolic pathway was not well known. β-sitosterol was assessed in vitro and in vivo using a dexamethasone-induced muscle atrophy mice model and C2C12 myoblasts. β-sitosterol protected mice from dexamethasone-induced muscle mass loss. The thickness of gastrocnemius muscle myofibers was increased in dexamethasone with the β-sitosterol treatment group (DS). Grip strength and creatine kinase (CK) activity were also recovered when β-sitosterol was treated. The muscle loss inhibitory efficacy of β-sitosterol in dexamethasone-induced muscle atrophy in C2C12 myotube was also verified in C2C12 myoblast. β-sitosterol also recovered the width of myotubes. The protein expression of muscle atrophy F-box (MAFbx) was increased in dexamethasone-treated animal models and C2C12 myoblast, but it was reduced when β-sitosterol was treated. MuRF1 also showed similar results to MAFbx in the mRNA level of C2C12 myotubes. In addition, in the gastrocnemius and tibialis anterior muscles of mouse models, Forkhead Box O1 (FoxO1) protein was increased in the dexamethasone-treated group (Dexa) compared with the control group and reduced in the DS group. Therefore, β-sitosterol would be a potential treatment agent for aging sarcopenia.
Keywords: FoxO1; MAFbx; MuRF1; dexamethasone; muscle atrophy; β-sitosterol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lipovec N.C., Schols A.M., Borst B.V.D., Beijers R.J., Kosten T., Omersa D., Lainscak M. Sarcopenia in Advanced COPD Affects Cardiometabolic Risk Reduction by Short-Term High-intensity Pulmonary Rehabilitation. J. Am. Med. Dir. Assoc. 2016;17:814–820. doi: 10.1016/j.jamda.2016.05.002. - DOI - PubMed
-
- Kang S.-Y., Lim G.E., Kim Y.K., Kim H.W., Lee K., Park T.-J., Kim J. Association between Sarcopenic Obesity and Metabolic Syndrome in Postmenopausal Women: A Cross-sectional Study Based on the Korean National Health and Nutritional Examination Surveys from 2008 to 2011. J. Bone Metab. 2017;24:9–14. doi: 10.11005/jbm.2017.24.1.9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

