A Digital Therapeutic Allowing a Personalized Low-Glycemic Nutrition for the Prophylaxis of Migraine: Real World Data from Two Prospective Studies
- PMID: 35889884
- PMCID: PMC9315551
- DOI: 10.3390/nu14142927
A Digital Therapeutic Allowing a Personalized Low-Glycemic Nutrition for the Prophylaxis of Migraine: Real World Data from Two Prospective Studies
Abstract
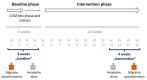
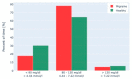
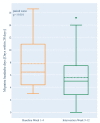
Migraine is a headache disorder associated with a high socioeconomic burden. The digital therapeutic sinCephalea provides an individualized low-glycemic diet based on continuous glucose measurement and is intended to provide a non-pharmacological migraine prophylaxis. We performed two prospective studies with migraine patients who used sinCephalea over a period of 16 weeks. The patients used a headache diary and recorded their migraine-related daily life impairments using the assessment tools HIT-6 and MIDAS for a pre versus post comparison. In addition, continuous glucose data of patients were compared to healthy controls. In both studies, patients reported a reduction of headache and migraine days as well as reductions in HIT-6 and MIDAS scores. More specifically, migraine days decreased by 2.40 days (95% CI [-3.37; -1.42]), HIT-6 improved by 3.17 points (95% CI [-4.63; -1.70]) and MIDAS by 13.45 points (95% CI [-22.01; -4.89]). Glucose data suggest that migraine patients have slightly increased mean glucose values compared to healthy controls, but drop into a glucose range that is below one's individual standard range before a migraine attack. In conclusion, sinCephalea is a non-pharmacological, digital migraine prophylaxis that induces a therapeutic effect within the range of pharmacological interventions.
Keywords: continuous glucose measurement; digital therapeutic; episodic migraine; headache; low-glycemic diet; low-glycemic index; migraine prophylaxis; nutrition; personalized nutrition; real world data.
Conflict of interest statement
F.S., O.W., G.K., D.K. and T.S. are/were employed at Perfood GmbH. T.S. and C.S. are co-founders of Perfood GmbH and minority shareholders. A.G., S.E., C.G. and D.T. received consulting fees from Perfood. The views presented in this manuscript are those of the authors and not necessarily those of Perfood GmbH. Good publication practices were followed. C.G. has received honoraria for consulting and lectures within the past three years from Allergan Pharma, Lilly, Novartis Pharma, Hormosan Pharma, Grünenthal, Sanofi-Aventis, Weber & Weber, Lundbeck, Perfood, and TEVA. His research is supported by a grant of the German Research Foundation (DFG). He does not hold any stocks of pharmaceutical companies. He is honorary secretary of the German Migraine and Headache Society. S.E. has received honoraria for consulting and lectures within the past three years from Allergan, Lilly, Lundbeck, Novartis, and Teva. D.T.: research support/principal investigator (clinical trials): AbbVie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Dermira, Eli Lilly, Galderma, GSK, Janssen-Cilag, Leo-Pharma, Novartis, Pfizer, Regeneron, Roche, Sandoz-Hexal, Sanofi, and UCB; consultant: AbbVie, Almirall, Amgen, Dignity, Galapagos, Leo Pharma, Maruho, Mitsubishi, Novartis, Sanofi, Pfizer, Regeneron, Target-Solution, and UCB; lectures: AbbVie, Almirall, Amgen, Janssen, Leo Pharma, MSD, Novartis, Pfizer, Roche-Posay, Sandoz-Hexal, Sanofi, and UCB; scientific advisory board: AbbVie, Boehringer Ingelheim, Eli Lilly, Galapagos, Janssen-Cilag, Leo Pharma, Morphosis, Novartis, Pfizer, Regeneron, Sanofi, and UCB. All other authors declare that they have no competing interests.
Figures
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- Yoon M.-S., Katsarava Z., Obermann M., Fritsche G., Oezyurt M., Kaesewinkel K., Katsarova A., Santowski I., Diener H., Moebus S. Prevalence of Primary Headaches in Germany: Results of the German Headache Consortium Study. J. Headache Pain. 2012;13:215–223. doi: 10.1007/s10194-012-0425-x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

