Quantification of Polyethylene Glycol 400 Excreted in the Urine by MALDI-TOF Mass Spectrometry
- PMID: 35890237
- PMCID: PMC9322888
- DOI: 10.3390/pharmaceutics14071341
Quantification of Polyethylene Glycol 400 Excreted in the Urine by MALDI-TOF Mass Spectrometry
Abstract
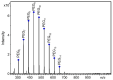
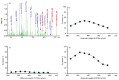
Polyethylene glycol 400 (PEG 400) was used as a permeability probe to examine the gastrointestinal tract which can be involved in the pathogenesis of some inflammatory and autoimmune diseases. A novel methodology was developed and validated for the quantitation of PEG 400 excreted in human urine after oral administration using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). The excretion ratios were determined for the most intense ions corresponding to nine PEG 400 oligomers. The relative error of accuracy was between -6.0% and 8.5%, and the relative standard deviation (RSD) of the precision was below 15%. Our method was successfully applied in a large-scale experimental study involving nearly two hundred volunteers. Due to the large number of measurements, detailed and reliable statistical analysis was performed. No significant difference was found between the male and female group of volunteers at 0.05 significance level, except the two largest PEG oligomers. However, the average excretion ratios of the male volunteers are greater than that of the women for all the nine PEG oligomers, suggesting a difference in the intestinal permeability between men and women.
Keywords: MALDI-TOF; intestinal permeability; polyethylene glycol; quantification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Relative quantification of polyethylene glycol 400 excreted in the urine of male and female volunteers by direct injection electrospray-selected ion monitoring mass spectrometry.Int J Pharm. 2011 Jul 29;414(1-2):35-41. doi: 10.1016/j.ijpharm.2011.04.061. Epub 2011 Apr 30. Int J Pharm. 2011. PMID: 21557993
-
Evaluation of the quantitativeness of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using an equimolar mixture of uniform poly(ethylene glycol) oligomers.J Mass Spectrom. 2003 Sep;38(9):948-54. doi: 10.1002/jms.508. J Mass Spectrom. 2003. PMID: 14505322
-
Direct identification of urinary tract pathogens from urine samples by matrix-assisted laser desorption ionization-time of flight mass spectrometry.J Clin Microbiol. 2010 Jun;48(6):2110-5. doi: 10.1128/JCM.02215-09. Epub 2010 Apr 14. J Clin Microbiol. 2010. PMID: 20392910 Free PMC article.
-
Current Status of Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometry (MALDI-TOF MS) in Clinical Diagnostic Microbiology.Molecules. 2020 Oct 17;25(20):4775. doi: 10.3390/molecules25204775. Molecules. 2020. PMID: 33080897 Free PMC article. Review.
-
Analysis of protein glycation products by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.Curr Med Chem. 2004 Aug;11(16):2185-93. doi: 10.2174/0929867043364649. Curr Med Chem. 2004. PMID: 15279557 Review.
Cited by
-
Incorporating a Polyethyleneglycol Linker to Enhance the Hydrophilicity of Mitochondria-Targeted Triphenylphosphonium Constructs.Chembiochem. 2023 Jun 1;24(11):e202200774. doi: 10.1002/cbic.202200774. Epub 2023 May 4. Chembiochem. 2023. PMID: 36917207 Free PMC article.
References
-
- Turecek P.L., Siekmann J. 4—PEG–protein conjugates: Nonclinical and clinical toxicity considerations. In: Pasut G., Zalipsky S., editors. Polymer-Protein Conjugates. Elsevier; Amsterdam, The Netherlands: 2020. pp. 61–101. - DOI
-
- Webster R., Elliott V., Park B.K., Walker D., Hankin M., Taupin P. PEG and PEG conjugates toxicity: Towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals. In: Veronese F.M., editor. PEGylated Protein Drugs: Basic Science and Clinical Applications. Birkhäuser Basel; Basel, Switzerland: 2009. pp. 127–146. - DOI
-
- Pascal F. The central role of excipients in drug formulation. Eur. Pharm. Rev. 2013;18:67–70.
-
- Daniela H. Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod. Chem. Appl. 2014;2:1–6.
-
- Bunker A. Poly(Ethylene Glycol) in Drug Delivery, Why Does it Work, and Can We do Better? All Atom Molecular Dynamics Simulation Provides Some Answers. Phys. Procedia. 2012;34:24–33. doi: 10.1016/j.phpro.2012.05.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

