Bacterial Cellulose as Drug Delivery System for Optimizing Release of Immune Checkpoint Blocking Antibodies
- PMID: 35890247
- PMCID: PMC9316226
- DOI: 10.3390/pharmaceutics14071351
Bacterial Cellulose as Drug Delivery System for Optimizing Release of Immune Checkpoint Blocking Antibodies
Abstract
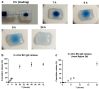
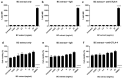
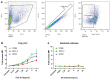
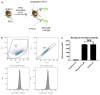
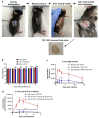
Immune checkpoint blocking therapy is a promising cancer treatment modality, though it has limitations such as systemic toxicity, which can often be traced to uncontrolled antibody spread. Controlling antibody release with delivery systems is, therefore, an attractive approach to reduce systemic antibody spread and potentially mitigate the side effects of checkpoint immunotherapy. Here, bacterial cellulose (BC) was produced and investigated as a delivery system for optimizing checkpoint-blocking antibody delivery. BC was produced in 24-well plates, and afterward, the edges were removed to obtain square-shaped BC samples with a surface of ~49 mm2. This customization was necessary to allow smooth in vivo implantation. Scanning electron microscopy revealed the dense cellulose network within BC. Human IgG antibody was included as the model antibody for loading and release studies. IgG antibody solution was injected into the center of BC samples. In vitro, all IgG was released within 24 to 48 h. Cell culture experiments demonstrated that BC neither exerted cytotoxic effects nor induced dendritic cell activation. Antibody binding assays demonstrated that BC does not hamper antibody function. Finally, antibody-loaded BC was implanted in mice, and serum measurements revealed that BC significantly reduced IgG and anti-CTLA-4 spread in mice. BC implantation did not induce side effects in mice. Altogether, BC is a promising and safe delivery system for optimizing the delivery and release of checkpoint-blocking antibodies.
Keywords: bacterial cellulose; cancer immunotherapy; checkpoint blocking therapy; drug delivery system; side effects; sustained release.
Conflict of interest statement
A.C. is affiliated to Percuros B.V. as a founder. D.K. is affiliated to JeNaCell GmbH as the CEO and as a founder. All authors declare no conflict of interests.
Figures

Similar articles
-
Thermosensitive hydrogels as sustained drug delivery system for CTLA-4 checkpoint blocking antibodies.J Control Release. 2020 Jul 10;323:1-11. doi: 10.1016/j.jconrel.2020.03.050. Epub 2020 Apr 2. J Control Release. 2020. PMID: 32247805
-
A versatile platform for the tumor-targeted delivery of immune checkpoint-blocking immunoglobin G.J Control Release. 2021 Dec 10;340:243-258. doi: 10.1016/j.jconrel.2021.11.003. Epub 2021 Nov 6. J Control Release. 2021. PMID: 34752799
-
Bacterial cellulose hydrogel loaded with lipid nanoparticles for localized cancer treatment.Colloids Surf B Biointerfaces. 2018 Oct 1;170:596-608. doi: 10.1016/j.colsurfb.2018.06.056. Epub 2018 Jun 26. Colloids Surf B Biointerfaces. 2018. PMID: 29975908
-
CTLA-4: As an Immunosuppressive Immune Checkpoint in Breast Cancer.Curr Mol Med. 2023;23(6):521-526. doi: 10.2174/1566524022666220610094716. Curr Mol Med. 2023. PMID: 35692146 Review.
-
Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade.Semin Oncol. 2010 Oct;37(5):430-9. doi: 10.1053/j.seminoncol.2010.09.005. Semin Oncol. 2010. PMID: 21074057 Review.
Cited by
-
Protein Immobilization on Bacterial Cellulose for Biomedical Application.Polymers (Basel). 2024 Aug 30;16(17):2468. doi: 10.3390/polym16172468. Polymers (Basel). 2024. PMID: 39274101 Free PMC article. Review.
-
Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose.Int J Mol Sci. 2023 Sep 27;24(19):14608. doi: 10.3390/ijms241914608. Int J Mol Sci. 2023. PMID: 37834056 Free PMC article. Review.
-
Recent advancements in development and application of microbial cellulose in food and non-food systems.Food Sci Biotechnol. 2024 Mar 23;33(7):1529-1540. doi: 10.1007/s10068-024-01524-0. eCollection 2024 Jun. Food Sci Biotechnol. 2024. PMID: 38623437 Free PMC article. Review.
-
Immune checkpoint blocking in cancer therapy using thermosensitive hydrogels: a review.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 2. doi: 10.1007/s00210-025-04171-2. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40314764 Review.
References
-
- Wei S.C., Anang N.A.S., Sharma R., Andrews M.C., Reuben A., Levine J.H., Cogdill A.P., Mancuso J.J., Wargo J.A., Pe’er D., et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl. Acad. Sci. USA. 2019;116:22699–22709. doi: 10.1073/pnas.1821218116. - DOI - PMC - PubMed
-
- Martins F., Sofiya L., Sykiotis G.P., Lamine F., Maillard M., Fraga M., Shabafrouz K., Ribi C., Cairoli A., Guex-Crosier Y., et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

