Another Move towards Bicalutamide Dissolution and Permeability Improvement with Acetylated β-Cyclodextrin Solid Dispersion
- PMID: 35890367
- PMCID: PMC9316079
- DOI: 10.3390/pharmaceutics14071472
Another Move towards Bicalutamide Dissolution and Permeability Improvement with Acetylated β-Cyclodextrin Solid Dispersion
Abstract
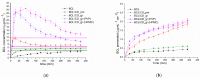
The complex formation of antiandrogen bicalutamide (BCL) with methylated (Me-β-CD) and acetylated (Ac-β-CD) β-cyclodextrins was investigated in buffer solution pH 6.8. A two-fold strongly binding of BCL to Ac-β-CD as compared to Me-β-CD was revealed. The solid dispersion of BCL with Ac-β-CD was prepared by the mechanical grinding procedure to obtain the complex in the solid state. The BCL/Ac-β-CD complex was characterized by DSC, XPRD, FTIR, and SEM techniques. The effect of Ac-β-CD in the BCL solid dispersions on the non-sink dissolution/permeation simultaneous processes was disclosed using the side-by-side diffusion cell with the help of the cellulose membrane. The elevated dissolution of the ground complex, as compared to the raw drug as well as the simple physical mixture, accompanied by the supersaturation was revealed. Two biopolymers-polyvinylpyrrolidone (PVP, Mn = 58,000) and hydroxypropylmethylcellulose (HPMC, Mn ~ 10,000)-were examined as the precipitation inhibitors and were shown to be useful in prolonging the supersaturation state. The BCL/Ac-β-CD complex has the fastest dissolution rate in the presence of HPMC. The maximal concentration of the complex was achieved at a time of 20, 30, and 90 min in the pure buffer, with PVP and with HPMC, respectively. The effectiveness of the BCL dissolution (release) processes (illustrated by the AUCC(t) parameter) was estimated to be 7.8-, 5.8-, 3.0-, and 1.8-fold higher for BCL/Ac-β-CD (HPMC), BCL/Ac-β-CD (PVP), BCL/Ac-β-CD (buffer), and the BCL/Ac-β-CD physical mixture, respectively, as compared to the BCL_raw sample. The excipient gain factor (EGF), calculated for the dissolution of the BCL complex, was shown to be 2.6 in the presence of HPMC, which is 1.3-fold greater as compared to PVP. From the experimental dissolution results, it can be concluded that the formation of BCL ground complex with Ac-β-CD enhances the dissolution rate of the compound. The permeation was also shown to be advantageous in the presence of the polymers, which was demonstrated by the elevated fluxes of BCL through the membrane. The comparison of the dissolution/permeation processes was illustrated and discussed. The conclusion was made that the presence of HPMC as a stabilizer of the supersaturation state is promising and seems to be a useful tool for the optimization of BCL pharmaceutical formulations manufacturing.
Keywords: complexation; dissolution; permeability; supersaturation; β-cyclodextrin derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Fischer J., Ganellin C.R. Analogue-Based Drug Discovery. John Wiley & Sons; Hoboken, NJ, USA: 2006. p. 515.
-
- Boers J., Venema C.M., de Vries E.F.J., Hospers G.A.P., Boersma H.H., Rikhof B., Dorbritz C., Glaudemans A.W.J.M., Schröder C.P. Serial [18F]-FDHT-PET to predict bicalutamide efficacy in patients with androgen receptor positive metastatic breast cancer. Eur. J. Cancer. 2021;144:151–161. doi: 10.1016/j.ejca.2020.11.008. - DOI - PubMed
LinkOut - more resources
Full Text Sources

