A Physiologically Based Pharmacokinetic and Pharmacodynamic Model of the CYP3A4 Substrate Felodipine for Drug-Drug Interaction Modeling
- PMID: 35890369
- PMCID: PMC9322514
- DOI: 10.3390/pharmaceutics14071474
A Physiologically Based Pharmacokinetic and Pharmacodynamic Model of the CYP3A4 Substrate Felodipine for Drug-Drug Interaction Modeling
Abstract
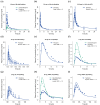
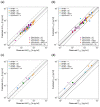
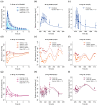
The antihypertensive felodipine is a calcium channel blocker of the dihydropyridine type, and its pharmacodynamic effect directly correlates with its plasma concentration. As a sensitive substrate of cytochrome P450 (CYP) 3A4 with high first-pass metabolism, felodipine shows low oral bioavailability and is susceptible to drug-drug interactions (DDIs) with CYP3A4 perpetrators. This study aimed to develop a physiologically based pharmacokinetic/pharmacodynamic (PBPK/PD) parent-metabolite model of felodipine and its metabolite dehydrofelodipine for DDI predictions. The model was developed in PK-Sim® and MoBi® using 49 clinical studies (94 plasma concentration-time profiles in total) that investigated different doses (1-40 mg) of the intravenous and oral administration of felodipine. The final model describes the metabolism of felodipine to dehydrofelodipine by CYP3A4, sufficiently capturing the first-pass metabolism and the subsequent metabolism of dehydrofelodipine by CYP3A4. Diastolic blood pressure and heart rate PD models were included, using an Emax function to describe the felodipine concentration-effect relationship. The model was tested in DDI predictions with itraconazole, erythromycin, carbamazepine, and phenytoin as CYP3A4 perpetrators, with all predicted DDI AUClast and Cmax ratios within two-fold of the observed values. The model will be freely available in the Open Systems Pharmacology model repository and can be applied in DDI predictions as a CYP3A4 victim drug.
Keywords: cytochrome P450 3A4 (CYP3A4); drug–drug interactions (DDIs); felodipine; pharmacodynamics; physiologically based pharmacokinetic (PBPK) modeling.
Conflict of interest statement
Thorsten Lehr has received research grants from the German Federal Ministry of Education and Research (grant 031L0161C). Felix Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219), and Deutsche Herzstiftung. He has received scientific support from Medronic and ReCor Medical and speaker honoraria from Astra-Zeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, and ReCor Medical. Laura Maria Fuhr, Fatima Zahra Marok, Maximilian Mees, and Dominik Selzer declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Pharmacokinetics of the CYP3A4 and CYP2B6 Inducer Carbamazepine and Its Drug-Drug Interaction Potential: A Physiologically Based Pharmacokinetic Modeling Approach.Pharmaceutics. 2021 Feb 17;13(2):270. doi: 10.3390/pharmaceutics13020270. Pharmaceutics. 2021. PMID: 33671323 Free PMC article.
-
Physiologically Based Pharmacokinetic (PBPK) Modeling of Clopidogrel and Its Four Relevant Metabolites for CYP2B6, CYP2C8, CYP2C19, and CYP3A4 Drug-Drug-Gene Interaction Predictions.Pharmaceutics. 2022 Apr 22;14(5):915. doi: 10.3390/pharmaceutics14050915. Pharmaceutics. 2022. PMID: 35631502 Free PMC article.
-
A Physiologically Based Pharmacokinetic Model of Ketoconazole and Its Metabolites as Drug-Drug Interaction Perpetrators.Pharmaceutics. 2023 Feb 17;15(2):679. doi: 10.3390/pharmaceutics15020679. Pharmaceutics. 2023. PMID: 36840001 Free PMC article.
-
PBPK modeling: What is the role of CYP3A4 expression in the gastrointestinal tract to accurately predict first-pass metabolism?CPT Pharmacometrics Syst Pharmacol. 2025 Jan;14(1):130-141. doi: 10.1002/psp4.13249. Epub 2024 Oct 2. CPT Pharmacometrics Syst Pharmacol. 2025. PMID: 39359052 Free PMC article.
-
Pharmacokinetic drug-drug interactions between 1,4-dihydropyridine calcium channel blockers and statins: factors determining interaction strength and relevant clinical risk management.Ther Clin Risk Manag. 2014;10:17-26. doi: 10.2147/TCRM.S55512. Epub 2013 Dec 20. Ther Clin Risk Manag. 2014. PMID: 24379677 Free PMC article. Review.
Cited by
-
Felodipine Promotes the Recovery of Mice With Spinal Cord Injury by Activating Macrolipophagy Through the AMPK-mTOR Pathway.J Cell Mol Med. 2025 Apr;29(8):e70543. doi: 10.1111/jcmm.70543. J Cell Mol Med. 2025. PMID: 40259510 Free PMC article.
-
Establishing Clinically Relevant Specifications for Carbamazepine Tablets Using Physiologically Based Pharmacokinetic Modeling.AAPS J. 2025 May 2;27(4):87. doi: 10.1208/s12248-025-01074-1. AAPS J. 2025. PMID: 40316811
-
Characterization of Drug with Good Glass-Forming Ability Loaded Mesoporous Silica Nanoparticles and Its Impact Toward in vitro and in vivo Studies.Int J Nanomedicine. 2024 Mar 6;19:2199-2225. doi: 10.2147/IJN.S453873. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38465205 Free PMC article. Review.
-
CYP3A4 and CYP3A5: the crucial roles in clinical drug metabolism and the significant implications of genetic polymorphisms.PeerJ. 2024 Dec 5;12:e18636. doi: 10.7717/peerj.18636. eCollection 2024. PeerJ. 2024. PMID: 39650550 Free PMC article. Review.
-
Development of a UPLC-MS/MS method for the determination of lacosamide and its metabolite and its application to drug-drug interaction.Front Pharmacol. 2023 Nov 10;14:1265252. doi: 10.3389/fphar.2023.1265252. eCollection 2023. Front Pharmacol. 2023. PMID: 38026954 Free PMC article.
References
-
- AstraZeneca LP. Plendil (Felodipine). Extended-Release Tablets. [(accessed on 26 October 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019834s025lbl.pdf.
-
- Heumann Pharma Felodipin Retard Heumann. [(accessed on 26 October 2021)]. Available online: https://www.heumann.de/fileadmin/user_upload/produkte/infos/Fachinformat....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

