HDAC Inhibition Regulates Cardiac Function by Increasing Myofilament Calcium Sensitivity and Decreasing Diastolic Tension
- PMID: 35890404
- PMCID: PMC9323146
- DOI: 10.3390/pharmaceutics14071509
HDAC Inhibition Regulates Cardiac Function by Increasing Myofilament Calcium Sensitivity and Decreasing Diastolic Tension
Abstract
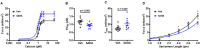
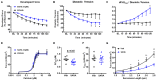
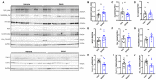
We recently established a large animal model that recapitulates key clinical features of heart failure with preserved ejection fraction (HFpEF) and tested the effects of the pan-HDAC inhibitor suberoylanilide hydroxamic acid (SAHA). SAHA reversed and prevented the development of cardiopulmonary impairment. This study evaluated the effects of SAHA at the level of cardiomyocyte and contractile protein function to understand how it modulates cardiac function. Both isolated adult feline ventricular cardiomyocytes (AFVM) and left ventricle (LV) trabeculae isolated from non-failing donors were treated with SAHA or vehicle before recording functional data. Skinned myocytes were isolated from AFVM and human trabeculae to assess myofilament function. SAHA-treated AFVM had increased contractility and improved relaxation kinetics but no difference in peak calcium transients, with increased calcium sensitivity and decreased passive stiffness of myofilaments. Mass spectrometry analysis revealed increased acetylation of the myosin regulatory light chain with SAHA treatment. SAHA-treated human trabeculae had decreased diastolic tension and increased developed force. Myofilaments isolated from human trabeculae had increased calcium sensitivity and decreased passive stiffness. These findings suggest that SAHA has an important role in the direct control of cardiac function at the level of the cardiomyocyte and myofilament by increasing myofilament calcium sensitivity and reducing diastolic tension.
Keywords: HDAC inhibitor; calcium; cardiomyocyte; contractility; heart failure; myofilament.
Conflict of interest statement
T.A.M. is on the SABs of Artemes Bio and Eikonizo Therapeutics, received funding from Italfarmaco for an unrelated project, and has a subcontract from Eikonizo Therapeutics for an SBIR grant from the National Institutes of Health (HL154959) J.A.K. received funding from Edgewise Therapeutics and Myokardia for unrelated projects.
Figures



Similar articles
-
HDAC inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction.Sci Transl Med. 2020 Jan 8;12(525):eaay7205. doi: 10.1126/scitranslmed.aay7205. Sci Transl Med. 2020. PMID: 31915304 Free PMC article.
-
Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction.Cardiovasc Res. 2013 Mar 1;97(3):464-71. doi: 10.1093/cvr/cvs353. Epub 2012 Dec 4. Cardiovasc Res. 2013. PMID: 23213108
-
Right-ventricular dysfunction in HFpEF is linked to altered cardiomyocyte Ca2+ homeostasis and myofilament sensitivity.ESC Heart Fail. 2021 Aug;8(4):3130-3144. doi: 10.1002/ehf2.13419. Epub 2021 May 17. ESC Heart Fail. 2021. PMID: 34002482 Free PMC article.
-
The Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid (SAHA) Restores Cardiomyocyte Contractility in a Rat Model of Early Diabetes.Int J Mol Sci. 2019 Apr 16;20(8):1873. doi: 10.3390/ijms20081873. Int J Mol Sci. 2019. PMID: 31014028 Free PMC article.
-
In vitro model to study the effects of matrix stiffening on Ca2+ handling and myofilament function in isolated adult rat cardiomyocytes.J Physiol. 2017 Jul 15;595(14):4597-4610. doi: 10.1113/JP274460. Epub 2017 Jun 21. J Physiol. 2017. PMID: 28485491 Free PMC article.
Cited by
-
Insights into posttranslational regulation of skeletal muscle contractile function by the acetyltransferases, p300 and CBP.J Appl Physiol (1985). 2024 Jun 1;136(6):1559-1567. doi: 10.1152/japplphysiol.00156.2024. Epub 2024 May 9. J Appl Physiol (1985). 2024. PMID: 38722753 Free PMC article.
-
Glycation in the cardiomyocyte.Vitam Horm. 2024;125:47-88. doi: 10.1016/bs.vh.2024.04.005. Epub 2024 May 24. Vitam Horm. 2024. PMID: 38997172 Free PMC article. Review.
-
Chromatin modifiers in human disease: from functional roles to regulatory mechanisms.Mol Biomed. 2024 Apr 8;5(1):12. doi: 10.1186/s43556-024-00175-1. Mol Biomed. 2024. PMID: 38584203 Free PMC article. Review.
References
-
- Cao D.J., Wang Z.V., Battiprolu P.K., Jiang N., Morales C.R., Kong Y., Rothermel B.A., Gillette T.G., Hill J.A. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc. Natl. Acad. Sci. USA. 2011;108:4123–4128. doi: 10.1073/pnas.1015081108. - DOI - PMC - PubMed
-
- Wallner M., Eaton D.M., Berretta R.M., Liesinger L., Schittmayer M., Gindlhuber J., Wu J., Jeong M.Y., Lin Y.H., Borghetti G., et al. HDAC inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction. Sci. Transl. Med. 2020;12:eaay7205. doi: 10.1126/scitranslmed.aay7205. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

