Cassava Frogskin Disease: Current Knowledge on a Re-Emerging Disease in the Americas
- PMID: 35890475
- PMCID: PMC9318364
- DOI: 10.3390/plants11141841
Cassava Frogskin Disease: Current Knowledge on a Re-Emerging Disease in the Americas
Abstract
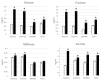
Cassava frogskin disease (CFSD) is a graft-transmissible disease of cassava reported for the first time in the 1970s, in Colombia. The disease is characterized by the formation of longitudinal lip-like fissures on the peel of the cassava storage roots and a progressive reduction in fresh weight and starch content. Since its first report, different pathogens have been identified in CFSD-affected plants and improved sequencing technologies have unraveled complex mixed infections building up in plants with severe root symptoms. The re-emergence of the disease in Colombia during 2019-2020 is again threatening the food security of low-income farmers and the growing local cassava starch industry. Here, we review some results obtained over several years of CFSD pathology research at CIAT, and provide insights on the biology of the disease coming from works on symptoms' characterization, associated pathogens, means of transmission, carbohydrate accumulation, and management. We expect this work will contribute to a better understanding of the disease, which will reflect on lowering its impact in the Americas and minimize the risk of its spread elsewhere.
Keywords: cassava; cassava frogskin disease; root yield; storage root; sugar content.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Pineda B., Jayasinghe U., Lozano J.C. La enfermedad ‘cuero de Sapo’ en yuca (Manihot esculenta Crantz) ASIAVA. 1983;4:10–12.
-
- Ceballos H. Chapter 1: Cassava in Colombia and the World: New Prospects for a Millennial Crop. In: Ospina B., Ceballos H., editors. Cassava in the Third Millenium: Modern Production, Processing, Use, and Marketing Systems. International Center for Tropical Agriculture (CIAT); Latin American and Caribbean Consortium to Support Cassava Research and Development (CLAYUCA); Technical Centre for Agricultural and Rural Cooperation (CTA); Cali, Colombia: 2012. pp. 1–11.
-
- Calvert L., Cuervo M., Lozano I. Chapter 16: Cassava viral disease in South America. In: Ospina B., Ceballos H., editors. Cassava in the Third Millenium: Modern Production, Processing, Use, and Marketing Systems. International Center for Tropical Agriculture (CIAT); Latin American and Caribbean Consortium to Support Cassava Research and Development (CLAYUCA); Technical Centre for Agricultural and Rural Cooperation (CTA); Cali, Colombia: 2012. pp. 309–321.
-
- Legg J., Kumar L., Makeshkumar T., Ferguson M., Kanju E., Ntawuruhunga P., Cuellar W.J. Cassava Virus Diseases: Biology, Epidemiology and Management. Adv. Virus Res. 2015;91:85–142. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

