Chitosan and Sodium Alginate Implementation as Pharmaceutical Excipients in Multiple-Unit Particulate Systems
- PMID: 35890597
- PMCID: PMC9316923
- DOI: 10.3390/polym14142822
Chitosan and Sodium Alginate Implementation as Pharmaceutical Excipients in Multiple-Unit Particulate Systems
Abstract
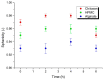
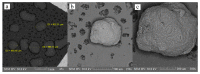
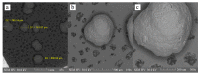
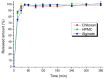
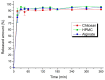
This study aimed to prepare and evaluate pellets containing acyclovir as a model drug. Pellets were prepared by the extrusion-spheronization process. Aqueous solutions of natural marine polymers (sodium alginate, chitosan) were compared to semi-synthetic hydroxypropyl methylcellulose (HPMC) in the role of binders. The study focused on the characterization of the pellet properties that are crucial for the formulation of the final dosage form, such as in multi-unit pellet system (MUPS) tablets or hard gelatin capsules filled with the pellets. Finally, the mentioned dosage forms were tested for drug dissolution. The morphology of pellets observed by scanning electron microscopy correlated with the shape evaluation performed by dynamic image analysis. Sodium alginate pellets exhibited the lowest value of sphericity (0.93), and many elongated rods and dumbbells were observed in this batch. Chitosan pellets had the highest value of sphericity (0.97) and were also less rough on the surface. The pellets maintained a constant surface geometry during the dissolution studies; they only reduced in size. The most significant reduction in size and weight was assessed after 2 h of dissolution testing. This fact was in line with the drug release from pellets in capsules or MUPS tablets, which was massive during the first hour, in both cases. The dissolution profiles of all of the batches were comparable.
Keywords: acyclovir; chitosan; hydroxypropyl methylcellulose; multi-unit pellet system; pellets; sodium alginate.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Hasnain M.S., Nayak A.K. Chapter 9—Chitosan as mucoadhesive polymer in drug delivery. In: Hasnain M.S., Beg S., Nayak A.K., editors. Chitosan in Drug Delivery. Academic Press; Cambridge, MA, USA: 2022. pp. 225–246. - DOI
-
- Barreiro-Iglesias R., Coronilla R., Concheiro A., Alvarez-Lorenzo C. Preparation of chitosan beads by simultaneous cross-linking/ insolubilisation in basic pH: Rheological optimisation and drug loading/release behaviour. Eur. J. Pharm. Sci. 2005;24:77–84. doi: 10.1016/j.ejps.2004.09.013. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

