Coordinative Chain Transfer Polymerization of Sustainable Terpene Monomers Using a Neodymium-Based Catalyst System
- PMID: 35890683
- PMCID: PMC9324384
- DOI: 10.3390/polym14142907
Coordinative Chain Transfer Polymerization of Sustainable Terpene Monomers Using a Neodymium-Based Catalyst System
Abstract
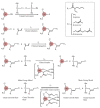
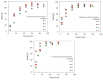
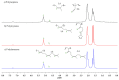
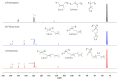
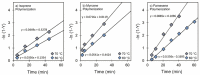
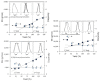
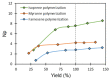
The present investigation involves the coordinative chain transfer polymerization (CCTP) of biobased terpenes in order to obtain sustainable polymers from myrcene (My) and farnesene (Fa), using the ternary Ziegler-Natta catalyst system comprising [NdV3]/[Al(i-Bu)2H]/[Me2SiCl2] and Al(i-Bu)2H, which acts as cocatalyst and chain transfer agent (CTA). The polymers were produced with a yield above 85% according to the monomeric consumption at the end of the reaction, and the kinetic examination revealed that the catalyst system proceeded with a reversible chain transfer mechanism in the presence of 15-30 equiv. of CTA. The resulting polyterpenes showed narrow molecular weight distributions (Mw/Mn = 1.4-2.5) and a high percent of 1,4-cis microstructure in the presence of 1 equiv. of Me2SiCl2, having control of the molecular weight distribution in Ziegler-Natta catalytic systems that maintain a high generation of 1,4-cis microstructure.
Keywords: biobased monomer; coordinative chain transfer polymerization; farnesene; myrcene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lei W., Zhou X., Russell T.P., Hua K., Yang X., Qiao H., Wang W., Li F., Wang R., Zhang L. High performance bio-based elastomers: Energy efficient and sustainable materials for tires. J. Mater. Chem. A. 2016;4:13058–13062. doi: 10.1039/C6TA05001H. - DOI
-
- Parcheta P.S., Głowińska E., Datta J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020;123:109422. doi: 10.1016/j.eurpolymj.2019.109422. - DOI
-
- Sarkar P., Bhowmick A.K. Synthesis, characterization and properties of a bio-based elastomer: Polymyrcene. RSC Adv. 2014;4:61343–61354. doi: 10.1039/C4RA09475A. - DOI
-
- Sahu P., Bhowmick A.K., Kali G. Terpene Based Elastomers: Synthesis, Properties, and Applications. Processes. 2020;8:553. doi: 10.3390/pr8050553. - DOI
-
- Sahu P., Sarkar P., Bhowmick A.K. Synthesis and Characterization of a Terpene-Based Sustainable Polymer: Poly-alloocimene. ACS Sustain. Chem. Eng. 2017;5:7659–7669. doi: 10.1021/acssuschemeng.7b00990. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

