Stability Program in Dendritic Cell Vaccines: A "Real-World" Experience in the Immuno-Gene Therapy Factory of Romagna Cancer Center
- PMID: 35891165
- PMCID: PMC9323699
- DOI: 10.3390/vaccines10070999
Stability Program in Dendritic Cell Vaccines: A "Real-World" Experience in the Immuno-Gene Therapy Factory of Romagna Cancer Center
Abstract
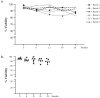
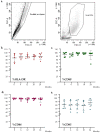
Advanced therapy medical products (ATMPs) are rapidly growing as innovative medicines for the treatment of several diseases. Hence, the role of quality analytical tests to ensure consistent product safety and quality has become highly relevant. Several clinical trials involving dendritic cell (DC)-based vaccines for cancer treatment are ongoing at our institute. The DC-based vaccine is prepared via CD14+ monocyte differentiation. A fresh dose of 10 million DCs is administered to the patient, while the remaining DCs are aliquoted, frozen, and stored in nitrogen vapor for subsequent treatment doses. To evaluate the maintenance of quality parameters and to establish a shelf life of frozen vaccine aliquots, a stability program was developed. Several parameters of the DC final product at 0, 6, 12, 18, and 24 months were evaluated. Our results reveal that after 24 months of storage in nitrogen vapor, the cell viability is in a range between 82% and 99%, the expression of maturation markers remains inside the criteria for batch release, the sterility tests are compliant, and the cell costimulatory capacity unchanged. Thus, the data collected demonstrate that freezing and thawing do not perturb the DC vaccine product maintaining over time its functional and quality characteristics.
Keywords: ATMP; dendritic cell vaccine; immunotherapy; quality control; stability.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
-
- Public Health EudraLex EudraLex—Volume 4—Good Manufacturing Practice (GMP) Guidelines. [(accessed on 5 November 2021)]. Available online: https://ec.europa.eu/health/documents/eudralex/vol-4_en.
-
- US FDA . Potency Tests for Cellular and Gene Therapy Products. Volume 27. US FDA; Silver Spring, MD, USA: 2011. pp. 568–577.
-
- European Medicines Agency Potency Testing of Cell-Based Immunotherapy Medicinal Products for the Treatment of Cancer. [(accessed on 8 November 2021)]; Available online: https://www.ema.europa.eu/en/potency-testing-cell-based-immunotherapy-me....
LinkOut - more resources
Full Text Sources
Research Materials

