GMMA as an Alternative Carrier for a Glycoconjugate Vaccine against Group A Streptococcus
- PMID: 35891202
- PMCID: PMC9324507
- DOI: 10.3390/vaccines10071034
GMMA as an Alternative Carrier for a Glycoconjugate Vaccine against Group A Streptococcus
Abstract
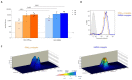
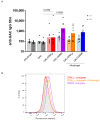
Group A Streptococcus (GAS) causes about 500,000 annual deaths globally, and no vaccines are currently available. The Group A Carbohydrate (GAC), conserved across all GAS serotypes, conjugated to an appropriate carrier protein, represents a promising vaccine candidate. Here, we explored the possibility to use Generalized Modules for Membrane Antigens (GMMA) as an alternative carrier system for GAC, exploiting their intrinsic adjuvant properties. Immunogenicity of GAC-GMMA conjugate was evaluated in different animal species in comparison to GAC-CRM197; and the two conjugates were also compared from a techno-economic point of view. GMMA proved to be a good alternative carrier for GAC, resulting in a higher immune response compared to CRM197 in different mice strains, as verified by ELISA and FACS analyses. Differently from CRM197, GMMA induced significant levels of anti-GAC IgG titers in mice also in the absence of Alhydrogel. In rabbits, a difference in the immune response could not be appreciated; however, antibodies from GAC-GMMA-immunized animals showed higher affinity toward purified GAC antigen compared to those elicited by GAC-CRM197. In addition, the GAC-GMMA production process proved to be more cost-effective, making this conjugate particularly attractive for low- and middle-income countries, where this pathogen has a huge burden.
Keywords: GMMA; Group A Carbohydrate; Group A Streptococcus; glycoconjugate.
Conflict of interest statement
This work was sponsored by GlaxoSmithKline Biologicals SA. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA. E.P., R.D.B., L.M., M.C., D.O., G.G., M.G.A., O.R., and F.M. (Francesca Micoli) are employees of the GSK group of companies. B.R. was also an employee of the GSK group of companies at the time of the experimental work. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Ralph A.P., Carapetis J.R. Group A Streptococcal Diseases and Their Global Burden. In: Chhatwal G.S., editor. Host-Pathogen Interactions in Streptococcal Diseases. Springer; Berlin/Heidelberg, Germany: 2013. pp. 1–27. - PubMed
-
- Watkins D.A., Johnson C.O., Colquhoun S.M., Karthikeyan G., Beaton A., Bukhman G., Forouzanfar M.H., Longenecker C.T., Mayosi B.M., Mensah G.A., et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N. Engl. J. Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

