Differential Dynamics of Humoral and Cell-Mediated Immunity with Three Doses of BNT162b2 SARS-CoV-2 Vaccine in Healthcare Workers in Japan: A Prospective Cohort Study
- PMID: 35891213
- PMCID: PMC9323262
- DOI: 10.3390/vaccines10071050
Differential Dynamics of Humoral and Cell-Mediated Immunity with Three Doses of BNT162b2 SARS-CoV-2 Vaccine in Healthcare Workers in Japan: A Prospective Cohort Study
Abstract
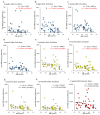
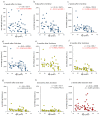
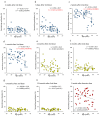
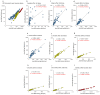
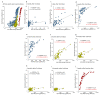
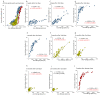
Vaccines against SARS-CoV-2 with good efficacy are now available worldwide. However, gained immunity diminishes over time. Here, we investigate the course of both humoral and cell-mediated immunity in response to three doses of the Pfizer mRNA BNT162b2 SARS-CoV-2 vaccine in healthcare workers in Japan. SARS-CoV-2 anti-receptor-binding domain (RBD) antibodies (total Ig, IgG), neutralizing antibodies (NAb), and ELISpot were measured in serum and whole blood samples collected after each vaccine dose. ELISpot numbers were higher than the cutoff values in most participants at all times. It was suggested that the difference in behavior between humoral immunity and cell-mediated immunity with age is complementary. Anti-RBD total Ig, IgG, and NAb indicated a high correlation at each time point after vaccine doses. Total Ig was retained long-term after the second dose and increased significantly faster by the booster dose than IgG. Nab levels of all subjects were ≤20% six months after the second dose, and the correlation coefficient was greatly reduced. These are due to the avidity of each antibody and differences among commercial kits, which may affect the evaluation of immunokinetics in previous COVID-19 studies. Therefore, it is necessary to harmonize reagents categorized by the same characteristics.
Keywords: BNT162b2 vaccine; ELISpot-; SARS-CoV-2; T-SPOT; anti-RBD antibody; antibody avidity; cell-mediated immunity; harmonize; humoral immunity.
Conflict of interest statement
M.M. has received grants provided to the Department of Laboratory Medicine, Hamamatsu University School of Medicine from Sysmex Co. Roche Diagnostics provided reagents for Cobas® SARS-CoV-2 antibodies assay kit and the IL-6 assay kit free of charge. Sysmex Co. provided the reagents for the HISCLTM SARS-CoV-2 antibodies assay kit, T-SPOT, free of charge. The other authors have no relevant financial interest in the products or companies described in this article.
Figures


References
-
- Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., Haljasmägi L., Rumm A.P., Maruste R., Kärner J., et al. Dynamics of Antibody Response to BNT162b2 Vaccine After Six Months: A Longitudinal Prospective Study. Lancet Reg. Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. - DOI - PMC - PubMed
-
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., COVID-19 Genomics UK (COG-UK) Consortium et al. SARS-CoV-2 Variants, Spike Mutations and Immune Escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

