A Novel Therapeutic Tumor Vaccine Targeting MUC1 in Combination with PD-L1 Elicits Specific Anti-Tumor Immunity in Mice
- PMID: 35891256
- PMCID: PMC9325010
- DOI: 10.3390/vaccines10071092
A Novel Therapeutic Tumor Vaccine Targeting MUC1 in Combination with PD-L1 Elicits Specific Anti-Tumor Immunity in Mice
Abstract
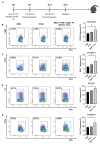
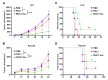
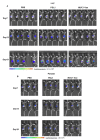
Dendritic cells (DCs), as professional antigen-presenting cells (APCs), play a key role in the initiation and regulation of humoral and cellular immunity. DC vaccines loaded with different tumor-associated antigens (TAAs) have been widely used to study their therapeutic effects on cancer. A number of clinical trials have shown that DCs are safe as an antitumor vaccine and can activate certain anti-tumor immune responses; however, the overall clinical efficacy of DC vaccine is not satisfactory, so its efficacy needs to be enhanced. MUC1 is a TAA with great potential, and the immune checkpoint PD-L1 also has great potential for tumor treatment. Both of them are highly expressed on the surface of various tumors. In this study, we generated a novel therapeutic MUC1-Vax tumor vaccine based on the method of PD-L1-Vax vaccine we recently developed; this novel PD-L1-containing MUC1-Vax vaccine demonstrated an elevated persistent anti-PD-L1 antibody production and elicited a much stronger protective cytotoxic T lymphocyte (CTL) response in immunized mice. Furthermore, the MUC1-Vax vaccine exhibited a significant therapeutic anti-tumor effect, which significantly inhibited tumor growth by expressing a high MUC1+ and PD-L1+ level of LLC and Panc02 tumor cells, and prolonged the survival of cancer-bearing animals. Taken together, our study provides a new immunotherapy strategy for improving the cross-presentation ability of therapeutic vaccine, which may be applicable to pancreatic cancer, lung cancer and for targeting other types of solid tumors that highly express MUC1 and PD-L1.
Keywords: MUC1; PD-L1; dendritic cells; immunotherapy; tumor vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A Novel PD-L1-Containing MSLN Targeting Vaccine for Lung Cancer Immunotherapy.Front Immunol. 2022 Jun 20;13:925217. doi: 10.3389/fimmu.2022.925217. eCollection 2022. Front Immunol. 2022. PMID: 35795680 Free PMC article.
-
A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention.Cancers (Basel). 2019 Dec 1;11(12):1909. doi: 10.3390/cancers11121909. Cancers (Basel). 2019. PMID: 31805690 Free PMC article.
-
Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors.Cancer Immunol Immunother. 2018 Mar;67(3):445-457. doi: 10.1007/s00262-017-2095-7. Epub 2017 Dec 4. Cancer Immunol Immunother. 2018. PMID: 29204701 Free PMC article.
-
Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses.Acta Pharm Sin B. 2020 May;10(5):723-733. doi: 10.1016/j.apsb.2019.09.006. Epub 2019 Sep 25. Acta Pharm Sin B. 2020. PMID: 32528824 Free PMC article. Review.
-
Post-Translational Modifications in Tumor-Associated Antigens as a Platform for Novel Immuno-Oncology Therapies.Cancers (Basel). 2022 Dec 26;15(1):138. doi: 10.3390/cancers15010138. Cancers (Basel). 2022. PMID: 36612133 Free PMC article. Review.
Cited by
-
Single cell RNA-seq and bulk RNA-seq analysis identifies MUC1 as a key gene for lung adenocarcinoma to neuroendocrine transformation.Transl Lung Cancer Res. 2025 Mar 31;14(3):824-841. doi: 10.21037/tlcr-24-806. Epub 2025 Mar 27. Transl Lung Cancer Res. 2025. PMID: 40248742 Free PMC article.
-
Synthetic self-adjuvanted multivalent Mucin 1 (MUC1) glycopeptide vaccines with improved in vivo antitumor efficacy.MedComm (2020). 2024 Feb 9;5(2):e484. doi: 10.1002/mco2.484. eCollection 2024 Feb. MedComm (2020). 2024. PMID: 38344400 Free PMC article.
-
C3-Liposome Delivery of MUC1 Peptide and TLR Agonists Enhances Adaptive Immunity and Results in Sex-Based Tumor Growth Differences.Pharmaceutics. 2025 Apr 3;17(4):468. doi: 10.3390/pharmaceutics17040468. Pharmaceutics. 2025. PMID: 40284463 Free PMC article.
-
Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment.Cells. 2023 Oct 4;12(19):2404. doi: 10.3390/cells12192404. Cells. 2023. PMID: 37830618 Free PMC article. Review.
-
The multifaceted roles of mucins family in lung cancer: from prognostic biomarkers to promising targets.Front Immunol. 2025 Jun 27;16:1608140. doi: 10.3389/fimmu.2025.1608140. eCollection 2025. Front Immunol. 2025. PMID: 40655139 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

