Evaluation of Safety and Immunogenicity of BNT162B2 mRNA COVID-19 Vaccine in IBD Pediatric Population with Distinct Immune Suppressive Regimens
- PMID: 35891273
- PMCID: PMC9318731
- DOI: 10.3390/vaccines10071109
Evaluation of Safety and Immunogenicity of BNT162B2 mRNA COVID-19 Vaccine in IBD Pediatric Population with Distinct Immune Suppressive Regimens
Abstract
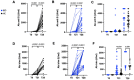
Patients affected by Inflammatory Bowel Disease (IBD) present higher risk for infection and suboptimal response upon vaccination. The immunogenicity of SARS-CoV2 vaccination is still largely unknown in adolescents or young adults affected by IBD (pIBD). We investigated the safety and immunogenicity of the BNT162B2 mRNA COVID-19 vaccine in 27 pIBD, as compared to 30 healthy controls (HC). Immunogenicity was measured by anti-SARS-CoV2 IgG (anti-S and anti-trim Ab) before vaccination, after 21 days (T21) and 7 days after the second dose (T28). The safety profile was investigated by close monitoring and self-reported adverse events. Vaccination was well tolerated, and short-term adverse events reported were only mild to moderate. Three out of twenty-seven patients showed IBD flare after vaccination, but no causal relationship could be established. Overall, pIBD showed a good humoral response upon vaccination compared to HC; however, pIBD on anti-TNFα treatment showed lower anti-S Ab titers compared to patients receiving other immune-suppressive regimens (p = 0.0413 at first dose and p = 0.0301 at second dose). These data show that pIBD present a good safety and immunogenicity profile following SARS-CoV-2 mRNA vaccination. Additional studies on the impact of specific immune-suppressive regimens, such as anti TNFα, on immunogenicity should be further investigated on larger cohorts.
Keywords: COVID-19 vaccine; IBD; pediatric.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Alatab S., Sepanlou S.G., Ikuta K., Vahedi H., Bisignano C., Safiri S., Sadeghi A., Nixon M.R., Abdoli A., Abolhassani H., et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. - DOI - PMC - PubMed
-
- Desalermos A., Pimienta M., Kalligeros M., Shehadeh F., Diamantopoulos L., Karamanolis G., Caldera F., Farraye F.A. Safety of Immunizations for the Adult Patient with Inflammatory Bowel Disease—A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2021:izab266. doi: 10.1093/ibd/izab266. - DOI - PubMed
-
- Pratt P.K., David N., Weber H.C., Little F.F., Kourkoumpetis T., Patts G.J., Weinberg J., Farraye F.A. Antibody Response to Hepatitis B Virus Vaccine Is Impaired in Patients with Inflammatory Bowel Disease on Infliximab Therapy. Inflamm. Bowel Dis. 2018;24:380–386. doi: 10.1093/ibd/izx001. - DOI - PubMed
-
- Fiorino G., Peyrin-Biroulet L., Naccarato P., Szabò H., Sociale O.R., Vetrano S., Fries W., Montanelli A., Repici A., Malesci A., et al. Effects of Immunosuppression on Immune Response to Pneumococcal Vaccine in Inflammatory Bowel Disease: A Prospective Study. Inflamm. Bowel Dis. 2012;18:1042–1047. doi: 10.1002/ibd.21800. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

