Advances in Infectious Disease Vaccine Adjuvants
- PMID: 35891284
- PMCID: PMC9316175
- DOI: 10.3390/vaccines10071120
Advances in Infectious Disease Vaccine Adjuvants
Abstract
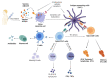
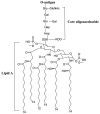
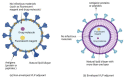
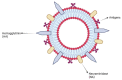
Vaccines are one of the most significant medical interventions in the fight against infectious diseases. Since their discovery by Edward Jenner in 1796, vaccines have reduced the worldwide transmission to eradication levels of infectious diseases, including smallpox, diphtheria, hepatitis, malaria, and influenza. However, the complexity of developing safe and effective vaccines remains a barrier for combating many more infectious diseases. Immune stimulants (or adjuvants) are an indispensable factor in vaccine development, especially for inactivated and subunit-based vaccines due to their decreased immunogenicity compared to whole pathogen vaccines. Adjuvants are widely diverse in structure; however, their overall function in vaccine constructs is the same: to enhance and/or prolong an immunological response. The potential for adverse effects as a result of adjuvant use, though, must be acknowledged and carefully managed. Understanding the specific mechanisms of adjuvant efficacy and safety is a key prerequisite for adjuvant use in vaccination. Therefore, rigorous pre-clinical and clinical research into adjuvant development is essential. Overall, the incorporation of adjuvants allows for greater opportunities in advancing vaccine development and the importance of immune stimulants drives the emergence of novel and more effective adjuvants. This article highlights recent advances in vaccine adjuvant development and provides detailed data from pre-clinical and clinical studies specific to infectious diseases. Future perspectives into vaccine adjuvant development are also highlighted.
Keywords: immunological adjuvants; infectious diseases; pre-clinical and clinical trials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

